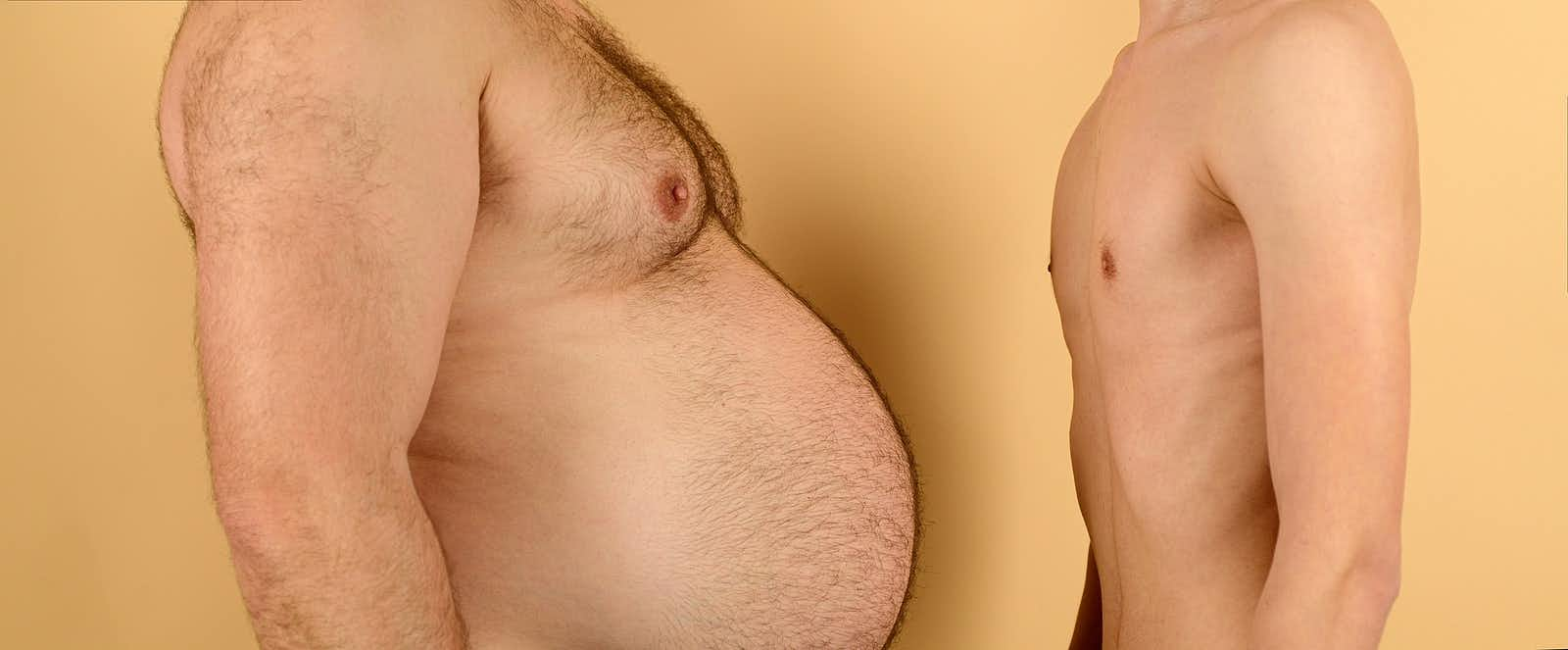
As you no doubt know, the buzz machine has been working overtime promoting weight loss drugs like Wegovy (semaglutide) and now Zepbound (tirzepatide). Zepbound is a twin of the diabetes drug Mounjaro, which has been in short supply because people have been using it off label to shed pounds. A new clinical trial shows that the drug also lowers blood pressure (Hypertension, Feb. 2024).
Tirzepatide for Hypertension?
In this study, 600 heavy people took tirzepetide for nine months. They did not have diabetes. The study was originally designed by Eli Lilly to investigate the ability of the medication to help people lose weight.
Researchers already knew that people taking tirzepatide have lower blood pressures in the doctor’s office. This trial took it even further and looked at continuous 24-hour blood pressure measurement. People on the drug had average systolic blood pressures 7 to 10 mm Hg lower than those on placebo.
That’s impressive! The results of the study:
“This study demonstrated the BP-lowering effects of tirzepatide in people with BMI ≥27 kg/m2, during both daytime and nighttime…This study demonstrates that tirzepatide improves 24-hour BP in obesity-related hypertension…Furthermore, nighttime systolic BP, which is a stronger predictor for cardiovascular death and all-cause death than daytime and 24-hour systolic BP, was also significantly reduced by tirzepatide. Correlation and mediation analyses indicated that tirzepatide-induced body weight reduction effects were associated with BP reductions, but tirzepatide, as a GLP-1/glucose-dependent insulinotropic polypeptide receptor agonist, may also have effects on BP independent of weight loss.”
That’s a bit in the weeds, but it is important. The researchers point out that losing weight can lead to reductions in blood pressure. But they also suggest that the drug might have a blood pressure-lowering effect “independent of weight loss.”
The authors conclude (Hypertension, Feb. 2024):
“These data provide further evidence for the potential benefits of tirzepatide on cardiometabolic health and cardiovascular outcomes.”
Getting Access to Tirzepatide:
Eli Lilly recently announced that it is launching a website service dubbed LillyDirect. It will connect patients to “telehealth service providers” around the country. We interpret that to mean patients will be connected to doctors who can prescribe a drug like Zepbound or Mounjaro. Then LillyDirect Pharmacy Solutions will facilitate the delivery of the medicine. The applecart has been turned upside down! What’s the skinny on the latest diabetes drug to get a green light for weight loss? Is Zepbound better than Wegovy when it comes to shedding pounds? What about side effects and cost?
A Paradigm Shift to Sell Zepbound:
When you think about getting a prescription for a new medicine, what comes to mind? Most people would say that 1) you first must make a doctor’s appointment. Then 2) you actually see a physician, PA or nurse practitioner. Once you have talked to a health professional and experienced a hands-on exam, 3) you get a prescription. It is either written on a piece of paper or more likely called in or sent electronically to a local pharmacy.
The idea that a drug manufacturer would facilitate a patient’s online doctor “appointment” or expedite the sale and distribution of a medicine direct to a consumer breaks the mold of the traditional doctor-patient and patient-pharmacist relationship.
The CEO of Lilly, David Ricks, told NBC News on January 4, 2024:
“‘We’re used to buying consumer goods directly from manufacturers all the time on online websites. It really hasn’t been an option that’s been provided before’ for prescription drugs.”
Are Drugs Just Like Other Consumer Goods Now?
I get the idea of buying sheets, headphones, books or batteries online. If you don’t like the product you can return it. No harm, no foul.
But are expensive medicines just like other consumer goods? It is unlikely you will be able to return a medicine if you change your mind or discover it causes unpleasant side effects. Try doing that at your local pharmacy. The pharmacist will tell you in no uncertain terms that returns are not accepted!
More concerning, though, is the amount of counseling you will get from an online health professional. Last time we checked, it is not possible to do a physical exam during a Zoom call. And how well will the online services follow up on your response to a drug like Zepbound? There are some serious side effects to be aware of.
A REALLY Short History of GLP-1 Agonists Against Diabetes and Obesity!
On November 8, 2023 the FDA approved Zepbound (tirzepatide) injections for the treatment of obesity. We saw this day coming back on August 3, 2023. That’s when we wrote an article titled:
Is the Diabetes Drug Mounjaro (Tirzepatide) Better Than Ozempic for Weight Loss?
Give me a moment to provide a short history of GLP-1 agonists. It’s not as boring as you might think.
Byetta:
The snowball started coming down the mountain back on April 28, 2005. That’s when the FDA approved exenatide (Byetta) to help control blood sugar.
Victoza:
On January 25, 2010, the injected drug liraglutide (Victoza) was also approved by the FDA for the treatment of type 2 diabetes. It too was a GLP-1 (glucagon-like peptide 1) agonist approved to control blood sugar.
Saxenda:
The same medication got the FDA’s green light for weight loss in December, 2014. The brand name was Saxenda. You can read more about this injectable medication and how well it worked at this link. Saxenda has to be injected daily. At the end of a year people lost about 12 pounds more than those getting placebo injections.
Ozempic:
In December, 2017, the FDA approved another GLP-1 agonist for the treatment of type 2 diabetes. The self-injectable drug semaglutide (Ozempic) was longer-acting than liraglutide. Instead of a daily shot, people could inject Ozempic once weekly.
Wegovy:
This drug also got a green light for weight loss, but not until June 4, 2021. Novo Nordisk gave semaglutide a shiny new name for weight loss. The new name was Wegovy when doctors prescribed it for obesity treatment. The snowball that started down the mountain so many years ago started picking up momentum!
It wasn’t until social media and Elon Musk that semaglutide injections really took off. You can read about that phenomenon at this link.
Mounjaro:
The FDA approved tirzepatide injections for the treatment of type 2 diabetes on May 13, 2022. The brand name was Mounjaro. It “activates” two receptors. The first is the old familiar GLP-1 (glucagon-like peptide 1). The second is a mouthful: glucose-dependent insulinotropic polypeptide (GIP). The goal of this dual-action, once-weekly injectable drug: improved blood sugar control. There are three different doses (5 milligrams, 10 mg and 15 mg).
Zepbound:
On November 8, 2023 the FDA announced the approval of tirzepatide injections for:
“…chronic weight management in adults with obesity (body mass index of 30 kilograms per square meter (kg/ m2) or greater) or overweight (body mass index of 27 kg/m2 or greater) with at least one weight-related condition (such as high blood pressure, type 2 diabetes or high cholesterol) for use, in addition to a reduced calorie diet and increased physical activity.”
Tirzepatide for Diabetes, Weight Loss and Hypertension?
We first wrote about tirzepatide (Zepbound) for weight loss on August 3, 2023. The title of that article was:
“Is the Diabetes Drug Mounjaro (Tirzepatide) Better Than Ozempic for Weight Loss?”
We were reporting on a press release from Eli Lilly. The drug company was reporting on the results of two clinical trials (SURMOUNT-3 and SURMOUNT-4) about tirzepatide for weight loss.
Here is what the company reported:
“Participants in SURMOUNT-3, after 12 weeks of intensive lifestyle intervention, achieved an additional 21.1% mean weight loss with tirzepatide for a total mean weight loss of 26.6% from study entry over 84 weeks
“Participants in SURMOUNT-4 achieved 21.1% weight loss during a 36-week tirzepatide lead-in period and an additional 6.7% weight loss during a 52-week continued treatment period, for a total mean weight loss of 26.0% over 88 weeks.”
How Does Zepbound Compare to Wegovy?
First, we rarely see head-to-head clinical trials of pharmaceuticals. Drug companies seem reluctant to test their brand name babies against competing medications. So, we cannot answer the question posed above in a perfectly scientific manner. To do so would require the exact same protocol for the drugs in question. Ideally, it would be carried out by a third party that had no financial interest in the outcome.
What we can say is that the FDA wrote this about Wegovy on June 4, 2021:
“The largest placebo-controlled trial enrolled adults without diabetes. The average age at the start of the trial was 46 years and 74% of patients were female. The average body weight was 231 pounds (105 kg) and average BMI was 38 kg/m2. Individuals who received Wegovy lost an average of 12.4% of their initial body weight compared to individuals who received placebo. Another trial enrolled adults with type 2 diabetes. The average age was 55 years and 51% were female. The average body weight was 220 pounds (100 kg) and average BMI was 36 kg/m2. In this trial, individuals who received Wegovy lost 6.2% of their initial body weight compared to those who received placebo.”
On November 8, 2023 the FDA wrote this about Zepbound:
“The larger of the two trials enrolled adults without diabetes. At the start of the trial, the average body weight was 231 pounds (105 kg) and average body mass index was 38 kg/m2. In this trial, those randomized to receive the highest approved dosage of Zepbound (15 mg once weekly) lost on average 18% of their body weight compared to those randomized to placebo.
“At the start of the trial in adults with type 2 diabetes, the average body weight was 222 pounds (101 kg) and average body mass index was 36 kg/m2. Those randomized to receive the highest approved dosage of Zepbound (15 mg once weekly) lost on average 12% of their body weight compared to those randomized to placebo.”
Relying on the FDA’s spin, it would appear that Wegovy helped overweight patients lose between 6.2% and 12.4% of their initial body weight compared to those on placebo. The agency reports that those on Zepbound lost 12% to 18% of their initial body weight compared to people getting placebo.
Eli Lilly Spins Zepbound Even More:
In its press release the drug company goes farther than the FDA in promoting Zepbound:
“Additionally, 1 in 3 patients taking Zepbound at the highest dose lost over 58 lb. (25% of body weight), compared to 1.5% on placebo, according to data not controlled for type 1 error. The average starting weight was 231 lb.”
Is Zepbound Safer Than Wegovy?
We are not ready to reach that conclusion. Here is what the drug company states in its press release.
“Warnings – Zepbound may cause tumors in the thyroid, including thyroid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath. If you have any of these symptoms, tell your healthcare provider.
“• Do not use Zepbound if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC).
• Do not use Zepbound if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
• Do not use Zepbound if you have had a serious allergic reaction to tirzepatide or any of the ingredients in Zepbound.”“Zepbound may cause serious side effects, including:
Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Zepbound. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
Kidney problems (kidney failure). Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration.
Gallbladder problems. Gallbladder problems have happened in some people who use Zepbound. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), or clay-colored stools.
Inflammation of the pancreas (pancreatitis). Stop using Zepbound and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Serious allergic reactions. Stop using Zepbound and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting or feeling dizzy, or very rapid heartbeat.
Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Zepbound with medicines that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include dizziness or light-headedness, sweating, confusion or drowsiness, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability, mood changes, hunger, weakness or feeling jittery.
Changes in vision in patients with type 2 diabetes. Tell your healthcare provider if you have changes in vision during treatment with Zepbound.
Depression or thoughts of suicide. You should pay attention to changes in your mood, behaviors, feelings or thoughts. Call your healthcare provider right away if you have any mental changes that are new, worse, or worry you.”
Common side effects
“The most common side effects of Zepbound include nausea, diarrhea, vomiting, constipation, stomach (abdominal) pain, indigestion, injection site reactions, feeling tired, allergic reactions, belching, hair loss, and heartburn. These are not all the possible side effects of Zepbound. Talk to your healthcare provider about any side effect that bothers you or doesn’t go away.
Tell your healthcare provider if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.”
Final Words about Zepbound:
Did your eyes glaze over about one tenth of the way down that list of side effects and warnings? It is easy to feel overwhelmed when faced with so many side effects. Drug companies seem more than willing to list a lot of potential adverse reactions.
I will not attribute motives to such long lists…but I suspect that many people zone out after reading about things like
“Multiple Endocrine Neoplasia syndrome type 2, medullary thyroid carcinoma, swelling of your face, lips, tongue, or stomach problems that are severe or will not go away.”
There may be one other reason for such long lists of side effects. If people are warned about possible adverse drug reactions in advance, they have a very hard time suing for damages if they suffer complications. As long as a pharmaceutical manufacturer warns about problems such as nausea, vomiting, diarrhea or death, it is hard to win a lawsuit. Read about this at this link.
What About Cost?
These drugs are pricey! According to Eli Lilly, Zepbound will cost around $1,000 for a month’s supply. That’s less than the list price of Wegovy, which is around $1,300 a month. Insurance companies may pay, but many people will have to meet pretty strict conditions to qualify.
Let’s be honest, drug companies are going to make a lot of money on GLP-1 and GIP agonists. Wegovy, Ozempic and Mounjaro have been in short supply because of the demand, despite the price.
Would you like a balanced perspective on the GLP-1 agonists like Wegovy and Zepbound? We recently interviewed two experts on obesity. We think it is worth spending a little time listening to our two-part series:
The Lowdown on New Medicines for Treating Obesity:
Here is Part 1:
Show 1361: The Lowdown on New Medicines for Treating Obesity
Prescribing new medicines for treating obesity has let our guest help many patients unable to lose weight by other means.
Here is Part 2:
Show 1362: Lowdown on New Medicines for Treating Obesity –Part 2
In this episode, we consider the benefits and considerable risks of drugs like Wegovy for treating obesity. Our guest says eat real food!
We welcome your reactions:
What do you think? Please share your thoughts about the “new” weight loss drugs in the comment section below. When you see the green box that states “View Comments,” please click. That will provide you with a sidebar listing Comments. You can add your thoughts in the box that says “Add your comment.”
We would also like to get your feedback on the new website LillyDirect. What do you think about the CEO of Lilly stating:
“‘We’re used to buying consumer goods directly from manufacturers all the time on online websites. It really hasn’t been an option that’s been provided before’ for prescription drugs.”
Do you consider medications just like other “consumer goods”? Do you think online doctors who can prescribe Zepbound should replace hands-on physician visits? What about pharmacists? Should drugstores go the way of the buggy whip, to be replaced by online pharmacy services that will deliver your Zepbound injectable drug to your mailbox or front door?
The last I checked, all these injectable medications are liquid. If you were in Iowa this week and the mailman or delivery person left your package in the mailbox or at the front door, it would freeze pretty fast. How will that work? The official prescribing information for Zebpound states:
- “Do not freeze ZEPBOUND. Do not use ZEPBOUND if frozen.”
The injectable drug is not supposed to be stored at temperatures over 86°F. If it is shipped to Phoenix in the summer, what do you want to guess it will exceed that temperature in the back of a delivery truck? The same thing could happen in a lot of states between July and August. Just wondering.
We would love to read your comments. Thank you for supporting The People’s Pharmacy.
Citations
- Jastreboff, A.M., et al, "Tirzepatide Once Weekly for the Treatment of Obesity," New England Journal of Medicine, July 21, 2022, DOI: 10.1056/NEJMoa2206038
- de Lemos, J.A., et al, "Tirzepatide Reduces 24-Hour Ambulatory Blood Pressure in Adults With Body Mass Index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy," Hypertension, Feb. 2024, DOI: 10.1161/HYPERTENSIONAHA.123.22022

