
Have you ever heard of authorized generic drugs? Most people, including some physicians and pharmacists, have no idea what they are. That’s largely because the Food and Drug Administration tells the world that all FDA approved generic drugs are equal to their brand name counterparts. But we have lost faith in the FDA’s ability to monitor generic drug manufacturing in distant lands. Can authorized generic drugs fill the void? At the bottom of this article you will find a list of authorized generic drugs.
Foreign Pharmaceutical Manufacturers:
Do you have any idea where your pills are made? Chances are good that you don’t. That’s because foreign drug companies have become adept at creating westernized names. They also set up offices in New Jersey and other states that make it seem as if the medications might be made in the US instead of in a foreign country.
Here is just one example: Prinston Pharmaceutical, Inc. has its headquarters in Somerset, NJ. Solco Healthcare U.S. is also located in Somerset, NJ and happens to be a “fully owned subsidiary” of Prinston Pharmaceutical, Inc. and Zhejiang Huahai Pharmaceuticals, headquartered in Zhejiang, China. You can read more about a problem with this company at this link.
The Latest FDA Concerns:
An article in FIERCE Pharma (Jan. 3, 2024) was titled:
“FDA slams trio of Indian drugmakers with Form 483 filings after December inspections”
According to the FDA:
“A Form FDA 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in their judgment may constitute violations of the Food, Drug, and Cosmetic (FD&C) Act and other Acts or regulations.”
In other words, when the FDA sends a Form FDA 483 to a drug company it is not good news.
FIERCE Pharma goes on to describe the problems FDA inspectors uncovered in India:
“Dr. Reddy’s Labs, Laurus Synthesis and Torrent Pharmaceuticals were each slammed with Form 483 filings from the FDA after the regulatory agency conducted a series of inspections in December.”
Dr. Reddy got into trouble because of “deficient records-keeping procedures.” It also failed on employee training and for “a lack of detailed control procedures used to review technical data.”
FIERCE Pharma reports that Torrent Pharmaceuticals in Gujarat, India got into trouble for failure to review batch discrepancies and “for not keeping equipment and tools clean enough.”
It also adds:
“Separately, in its Form 483, Laurus Synthesis was hit with four observations following a review of its Anakapalli manufacturing facility located in Andhra Pradesh, India. The observations included not adequately conducting investigations of unexpected occurrences, inadequate sampling plans for intermediates, and not maintaining, cleaning and storing equipment in a way to prevent contamination.
“Laurus was also cited for keeping incomplete records related to batch production and control.”
This is not the first time that drug companies in India have gotten into trouble. Here is a link that describes problems going back quite a long time.
What About Authorized Generic Drugs?
The FDA has to tell the American public about generic drugs. That’s because of the passage of the Food and Drug Administration Amendments Act (FDAAA) on September 27, 2007. Here is the FDA’s link to authorized generic drugs.
The only problem with this list is that it does not tell you the company that is actually distributing/selling the authorized generic drug. It only tells you the company that applied for the NDA (new drug application). That makes it hard for physicians, pharmacists or patients to know what company is actually selling the authorized generic drug for any particular brand name medicine.
Insurance Companies Embrace Generic Drugs:
Generic drugs are copycat versions of brand name medicines. That means they contain the same active ingredient. According to the Food and Drug Administration, all generic drugs are equally good as long as they have been approved by the agency.
But the generic company does not have access to the secret (proprietary) recipe that the brand name company used. In addition to different inactive ingredients (binders, fillers, dyes), the release formulation may be quite different. That means that the way the drug dissolves in your digestive tract can be quite different from the brand name. That was the problem with Budeprion XL 300, a generic form of Wellbutrin XL 300.
Generic Wellbutrin XL 300:
Insurance companies love generic drugs because they are so much less expensive than the brand name products. They often refuse to pay for brand names if there is a generic option available.
Take the antidepressant Wellbutrin XL 300, for example. The brand name costs between $1,700 and $2,000 for a month’s supply. The generic, bupropion XL 300, costs between $22 and $100 for the same amount, depending on the pharmacy.
It’s hardly any wonder that many insurance companies won’t pay for the brand name once the generic is on the market. But many readers of our syndicated newspaper column have reported that certain generic drugs do not appear to work as well as the brand name counterparts.
One person recently wrote:
“I feel the need to share my experience with the changes made to my antidepressant, Wellbutrin. I was prescribed Wellbutrin 300, and I’ve taken it for over 12 years. Two weeks ago, my pharmacy sent me a different type of bupropion XL.
“Since starting on this new generic, I have had the worst time ever. I feel so much rage! I am agitated and hate everyone and anything! How can this happen? I feel like I am in an escalating downward spin.
“Today I realized these feelings of rage began when I started taking this new bupropion. I called my doctor to inform her of my reactions and to enlist her assistance. She explained that many people react differently when pharmacies use a different manufacturer to fill prescriptions. I am so grateful that she listened and requested a change back to the original medication.”
Actually An Old Story!
This is not the first time we have heard of such problems with certain generic bupropion products. Starting early in 2007 we began hearing about problems with one particular generic form of bupropion (Budeprion XL 300). Here is a link to that article. https://www.peoplespharmacy.com/articles/side-effects-of/
We began lobbying the FDA to test this generic form of Wellbutrin. For years we were told there was no problem with the generic formulation. Any reports of side effects or lack of effectiveness were psychosomatic. In other words, they were all in the person’s head. We did not give up, though. We forwarded nasty smelling Budeprion pills that people sent us to the FDA. We kept badgering the FDA to do something. We believed that lives were at stake.
Finally, on October 3, 2012, the FDA announced that it would require the removal of generic Budeprion XL 300 because it was “not therapeutically equivalent to the reference listed drug (RLD, Wellbutrin XL 300 mg.” If you would like to read the saga of this generic drug boondoggle, here is a link.
Other Generic Drug Disasters:
Many generic drug companies abroad have gotten into trouble with the FDA. Most recently, the generic blood pressure pill valsartan was found to be contaminated with a probable carcinogen. The active pharmaceutical ingredient was produced in China. Read more about that debacle here.
If you examine the FDA’s oversight of foreign-made medicines, it leaves a lot to be desired. For one thing, FDA inspectors have to notify the generic drug companies in advance that they are coming. That would be a little like telling a restaurant owner that health department inspectors will be coming by to check things out in six weeks. It’s amazing that many generic drug makers still fail their inspections given such advance notice.
We have also determined that there are flaws in the FDA’s generic drug approval process. Instead of comparing the generic drug absorption hour by hour, the agency aggregates the data. We think this completely obscures the process. The best way to see what we mean is by looking at the bioequivalence curves for Budeprion compared to Wellbutrin.
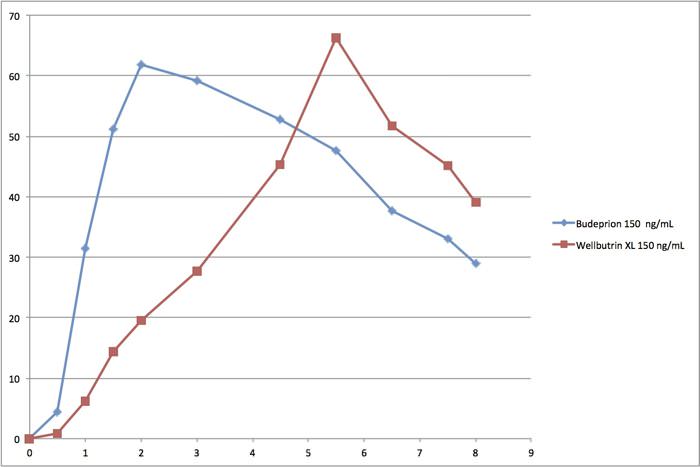
This is a chart of the first eight hours comparing Budeprion XL 150 to Wellbutrin XL 150. On the left is blood concentration ranging from 0 to 70 ng/ml (nanograms per ml). On the bottom is hours. It does not take a pharmacologist to realize that these curves are quite different. And yet this product remains on the market as bioequivalent even though the 300 mg version was deemed bioinequivalent. The FDA’s system for approving generic drugs confounds us!
What Are Authorized Generic Drugs?
Is there any way to take a generic drug that would offer a good price but still be of high quality? As it turns out, there is. You could seek out authorized generic drugs.
When a generic drug company makes a deal with the brand-name manufacturer to get an exclusive license, that is known as an authorized generic. In many instances, it is made in the same plant and on the same production line as the original brand-name medicine. Sometimes the generic company is a subsidiary of the brand name manufacturer.
If it is made somewhere else, the generic manufacturer must use the same recipe and the same inactive ingredients. The delivery system must also be the same for an authorized generic.
Unfortunately, many health professionals are not aware of the concept of authorized generic drugs. It may be difficult to get the pharmacy to fill your prescription with an authorized generic even if one is available. Large chain drug companies like to cut deals with specific generic drug manufacturers. Such authorized generics may cost a bit more than other generic products, but they are almost always considerably less than the brand.
Flovent–PBMs Block Access to the Authorized Generic Drug:
Flovent (fluticasone), a popular asthma drug, will soon disappear from the market. Do not despair, though. An authorized generic will soon become available.
Efforts to control drug prices through the Inflation Reduction Act sometimes have unexpected consequences. That appears to be the case with the very popular asthma inhaler called Flovent. The manufacturer, Glaxo SmithKline (GSK) just announced that it is taking Flovent off the market.
Instead, it will offer an authorized generic fluticasone HFA inhaler that will cost 35% less than the brand name product it has sold for more than 20 years. Because it will continue to be made on the same production line, consumers should pay less for the identical medicine.
This may seem like a good outcome for patients and insurance companies, but pharmacy-benefit managers (PBMs) have thrown a monkey wrench into the works. Many PBMs are refusing to cover the new authorized generic. That’s because it will probably cost more than other generic fluticasone inhalers.
Some pediatricians worry that children could find other inhalers hard to use. GSK will be keeping the exact same inhaler technology for its authorized generic.
Getting Access to Authorized Generic Drugs:
You can learn more about authorized generic drugs and get a list of selected manufacturers in our eGuide to Saving Money on Medicines. It is available at www.PeoplesPharmacy.com in our Health Guide Section.
Authorized Generic Drugs:
The following list contains the authorized generic drug name followed by the brand name inside the parenthesis followed by the manufacturer inside brackets. With this information your pharmacist should be able to locate the precise authorized generic drug that you seek.
- Abacavir and Lamivudine (Epzicom) [Prasco]
- Abiraterone Tablets (Zytiga) [Patriot]
- Acitretin (Soriatane) [Prasco]
- Adapalene and Benzoyl Peroxide Gel (Epiduo) [Prasco]
- Adapalene Gel (Differin) [Prasco]
- Alprazolam Tablets (Xanax) [Greenstone]
- Amlodipine and Atorvastatin (Caduet) [Greenstone]
- Atazanavir (Reyataz) [Greenstone]
- Atomoxetine (Strattera) [Prasco]
- Atorvastatin (Lipitor) [Greenstone]
- Atovaquone and Proguanil (Malarone) [Prasco]
- Atovaquone Suspension (Mepron) [Prasco]
- Augmented Betamethasone Dipropionate (Diprolene) [Prasco]
- Azithromycin Pak (Zithromax or Z-pak) [Greenstone]
- Cabergoline (Dostinex) [Greenstone]
- Calcipotriene Cream (Dovonex) [Prasco]
- Carbamazepine Extended-Release (Carbatrol) [Prasco]
- Celecoxib (Celebrex) [Greenstone]
- Clindamycin Benzoyl Peroxide Topical Gel (Duac) [Prasco]
- Clindamycin Capsules & Gels (Cleocin) [Greenstone]
- Clomiphene Tablets (Clomid)
- Clonidine Extended-Release Tablets (Kapvay) [Prasco]
- Clotrimazole and Betamethasone Dipropionate Cream (Lotrisone) [Prasco]
- Colchicine (Colcrys) [Prasco]
- Colestipol Granules, Tablets (Colestid) [Greenstone]
- Dactinomycin for Injection (Cosmegen) [Prasco]
- Dapsone Gel (Aczone) [Greenstone]
- Desvenlafaxine ER Tablets (Pristiq) [Greenstone]
- Diclofenac & Misoprostol Tablets (Arthrotec) [Greenstone]
- Diphenoxylate and Atropine (Lomotil) [Greenstone]
- Dofetilide (Tikosyn) [Greenstone]
- Doxazosin (Cardura) [Greenstone]
- Doxycycline 40 mg Capsules (Oracea) [Prasco]
- Doxycycline Hyclate Capsules (Vibramycin) [Greenstone]
- Dutasteride and Tamsulosin (Jalyn) [Prasco]
- Eletriptan (Relpax) [Greenstone]
- Eplerenone (Inspra) [Greenstone]
- Ethosuximide (Zarontin) [Greenstone]
- Exemestane (Aromasin) [Greenstone]
- Fluconazole for Oral Suspension (Diflucan) [Greenstone]
- Fluconazole Tablets (Diflucan) [Greenstone]
- Fluorometholone Ophthalmic (FML) [Greenstone]
- Fluticasone HFA Inhaler (Flovent) [Prasco]
- Fluticasone & Salmeterol (Advair) [Prasco]
- Galantamine Capsulres/Tablets (Razadyne) [Patriot]
- Gabapentin Oral Solution (Neurontin) [Greenstone]
- Gatifloxacin Ophthalmic Solution (Zymaxid) [Greenstone]
- Gentamicin Ophthalmic Solution (Genoptic) [Greenstone]
- Glipizide XL (Glucotrol XL) [Greenstone]
- Hydrocortisone Tablets (Cortef) [Greenstone]
- Hydroxychloroquine (Plaquenil) [Prasco]
- Hydroxyprogesterone Caproate Injection (Makena) [Prasco]
- Ibuprofen Lysine Injection (NeoProfen) [Prasco]
- Itraconazole Capsules & Oral Solution (Sporanox) [Patriot]
- Ketorolax Ophthalmic Solution (Acular LS) [Greenstone]
- Ketoconazole Shampoo (Nizoral Shampoo) [Patriot]
- Lamivudine Tablets (HBV) (Epivir-HBV) [Prasco]
- Lansoprazole, Amoxicillin, Clarithromycin (Prevpac) [Prasco]
- Lanthanum Carbonate Chewable Tablets (Fosrenol) [Prasco]
- Latanoprost Ophthalmic Solution (Xalatan) [Greenstone]
- Levalbuterol HCl Inhalation Solution (Xopenex) [Prasco]
- Levobunolol Ophthalmic Solution (Betagan) [Greenstone]
- Linezolid (Zyvox) [Greenstone]
- Medroxyprogesterone Tablets (Provera) [Greenstone]
- Mefenamic Acid (Ponstel) [Prasco]
- Mesalamine delayed-release tablets (Lialda) [Prasco]
- Methylprednisolone Tablets (Medrol) [Greenstone]
- Metronidazole Gel, 1% (Metrogel) [Prasco]
- Metronidazole Topical Lotion (MetroLotion) [Prasco]
- Miglustat Capsules (Zavesca) [Patriot]
- Misoprostol Tablets (Cytotec) [Greenstone]
- Mixed Amphetamine ER & XR (Adderall ER & XR) [Prasco]
- Montelukast Sodium Oral Granules (Singulair) [Prasco]
- Nadolol (Corgard) [Greenstone]
- Nifedipine (Procardia) [Greenstone]
- Nisoldipine Extended Release (Sular) [Prasco]
- Nitroglycerine Sublingual Tablets (Nitrostat) [Greenstone]
- Omega-3-acid ethyl esters Capsules (Lovaza) [Prasco]
- Oxaprozin (Daypro) [Greenstone]
- Oxybutynin Extended Release Tablets (Ditropan XL) [Patriot]
- Paliperidone Extended Release Tablets (Invega) [Patriot]
- Phenelzine (Nardil) [Greenstone]
- Phenoxybenzamine Capsules (Dibenzyline) [Prasco]
- Phenytoin Infatabs (Infatabs) [Greenstone]
- Phenytoin Oral Suspension & Tablets (Dilantin) [Greenstone]
- Pioglitazone and Glimepiride (Duetact) [Prasco]
- Piroxicam (Feldene) [Greenstone]
- Polymyxin and Trimethoprim Ophthalmic (Polytrim) [Greenstone]
- Prasugrel Tablets (Effient) [Prasco]
- Prazosin (Minipress) [Greenstone]
- Prednisolone Ophthalmic (Pred Forte) [Greenstone]
- Prednisolone Sodium Phosphate Disintegrating Tablets (Orapred) [Prasco]
- Propafenone HCl ER (Rythmol SR) [Prasco]
- Quinapril & Hydrochlorothiazide (Accuretic) [Greenstone]
- Rifabutin (Mycobutin) [Greenstone]
- Risedronate Delayed-Release (Atelvia) [Greenstone]
- Risedronate Tablets (Actonel) [Greenstone]
- Risperidone (Risperdal) [Patriot]
- Sertraline Oral Solution (Zoloft) [Greenstone]
- Sildenafil (Viagra) [Greenstone]
- Silver Sulfadiazine Cream (Silvadine) [Greenstone]
- Sirolimus (Rapamune) [Greenstone]
- Spironolactone & Hydrochlorothiazide (Aldactazide) [Greenstone]
- Sucralfate (Carafate) [Greenstone]
- Sulfacetamide Ophthalmic (Bleph-10) [Greenstone]
- Sulfasalazine Delayed-Release (Azulfidine EN-Tabs) [Greenstone]
- Sulfasalazine Tablets (Azulfidine) [Greenstone]
- Tadalafil Tablets (Cialis) [Prasco]
- Tazarotene Cream (Tazorac) [Greenstone]
- Testosterone Gel (Testim) [Prasco]
- Tolterodine ER Capsules (Detrol LA) [Greenstone]
- Tolterodine Tablets (Detrol) [Greenstone]
- Trandolapril Verapamil (Tarka) [Greenstone]
- Triazolam (Halcion) [Greenstone]
- Voriconazole (Vfend) [Greenstone]
- Zileuton ER Tablets (Zyflo CR) [Prasco]
- Ziprasidone (Geodon) [Greenstone]
Share your Story:
Please tell us your experience with generic drugs. Have you ever tried an authorized generic? Did it make a difference?

