Common Culprits
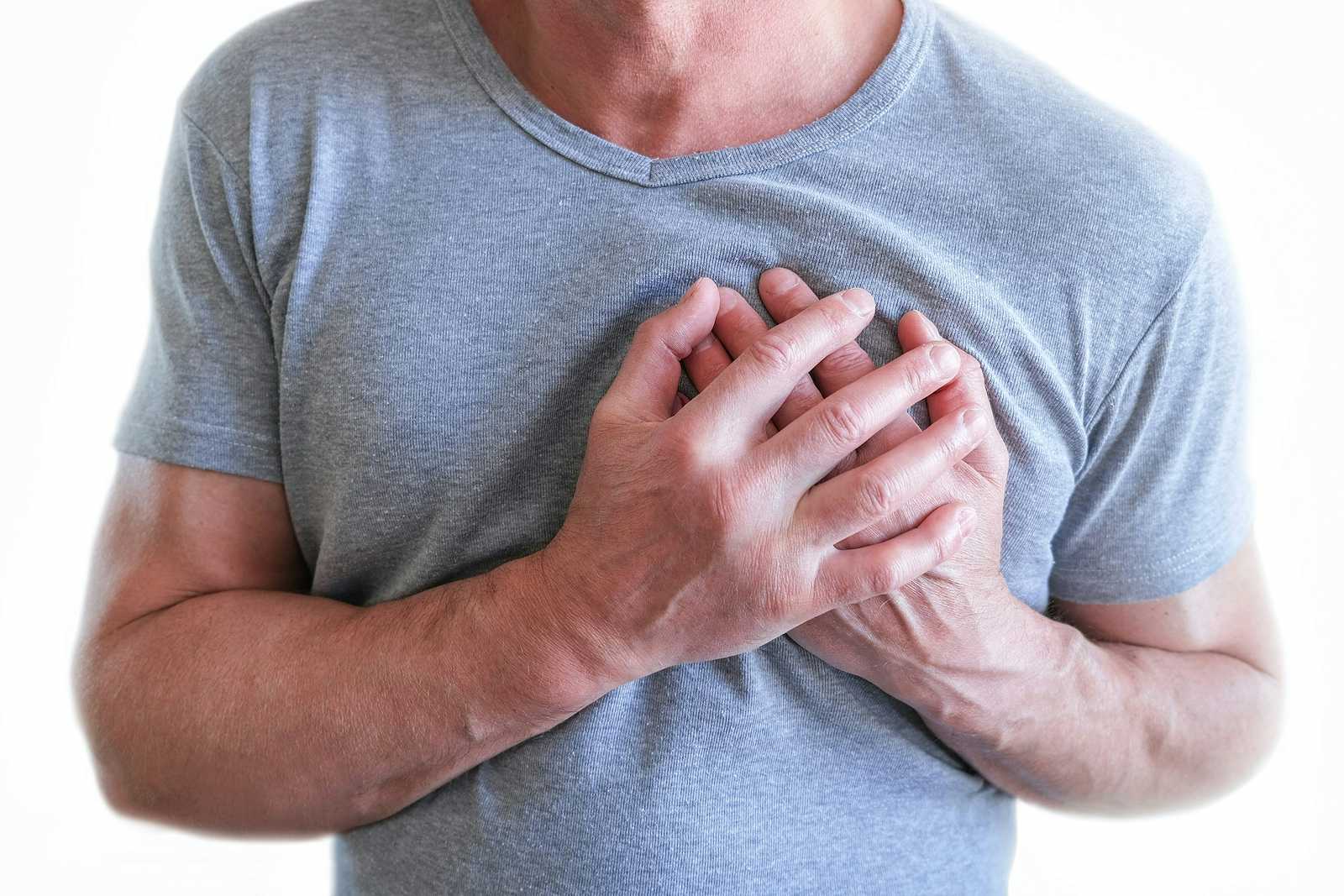
When doctors talk about infections, they are usually referring to acute situations in which the immune system gets overwhelmed by a virus such as influenza or chickenpox. Infections also result from the interaction of bacteria with the immune system, as in the case of pneumonia or sepsis. These can be crises, but they are relatively short-lived, resolving one way or the other within a few weeks or at most months. Could infections trigger chronic diseases? Our guest, evolutionary biologist Dr. Paul Ewald, thinks they do.
At The People’s Pharmacy, we strive to bring you up to date, rigorously researched insights and conversations about health, medicine, wellness and health policies and health systems. While these conversations intend to offer insight and perspective, the content is provided solely for informational and educational purposes. Please consult your healthcare provider before making any changes to your medical care or treatment.
How You Can Listen:
You could listen through your local public radio station or get the live stream at 7 am EST on Saturday, Dec. 13, 2025, through your computer or smart phone (wunc.org). Here is a link so you can find which stations carry our broadcast. If you can’t listen to the live broadcast, you may wish to hear the podcast later. You can subscribe through your favorite podcast provider, download the mp3 using the link at the bottom of the page, or listen to the streaming audio on this post starting on Dec. 15, 2025. It can be found under the photo at the top of the page.
How Infections Trigger Chronic Diseases:
Investigating the origins of chronic diseases requires a great deal of patience and the ability to examine several different areas that might be relevant. Over the past few decades, the technology for evaluating genetic contributions has improved greatly. What we have learned is that most chronic conditions are associated with a range of genes that each add a small amount of risk.
To get further insight, we have to look at the environment. This broad area includes topics as far ranging as sunshine, stress and nutrition. In particular, we need to look at the pathogens present in any given environment, as they could play an important role in our health.
Scrutinizing the environment is not enough. To understand the impact on disease, we need to know more about human behavior within that environment. How much sun exposure do the patients get? Are they sleeping? Where do they spend most of their time, and with whom? These all will help us understand the link to pathogens.
What We Have Learned About the Microbiome:
Over the past several decades, scientists have learned a great deal about the microbiome. The original conception of gut bacteria has been enriched with the understanding that almost every part of the human body has its own microbiome, almost as unique as a fingerprint. These collections of microbes live in harmony–or disequilibrium–with microbes from the environment. Some of these may be beneficial. Others undoubtedly are harmful, and we call them pathogens. How do pathogens trigger chronic diseases?
How Does the Body React to Pathogens?
When pathogens are detected, the immune system responds. Often, that comes in the form of macrophages, immune cells that circulate in the blood and attack the pathogens. Even a type of microbe that normally cohabits peacefully with the others in its space can cause trouble if it becomes too numerous or goes out of bounds. One example is Porphyromonas gingivalis. It’s usually found in the mouth. If it gets too exuberant there, it can cause gum disease. Worse, though, the macrophages dispatched to deal with P. ginigivalis anywhere in the body can end up collecting in atherosclerotic plaque in arteries (Signal Transduction and Targeted Therapy, May 23, 2025).
Another example of pathogens causing unexpected trouble is Clostridium (or Clostridioides) difficile (C. diff). These bacteria can live among other gut microbes and you might not even know they were there. But if the microbiota become disturbed, from a course of antibiotic treatment, for example, C. diff can proliferate and cause terrible diarrhea that may be very difficult to treat. Studies indicate that C. diff has evolved so that the strains in hospitals are now more likely to be resistant to antibiotic medications.
Alzheimer disease seems like a chronic condition rather than a complication of infection. Certainly, researchers have been examining genetic predispositions for the accumulation of beta-amyloid plaque in the brain. Yet Alzheimer disease is associated with microbes such as Chlamydia pneumoniae and P. gingivalis. Could flossing your teeth to reduce your chance of periodontal disease also help lower your risk of Alzheimer disease? Recent research has shown that older people receiving the shingles vaccine are less likely to be diagnosed with dementia. Perhaps amyloid plaques in the brain are part of an immune response to infection.
Has Long COVID Shifted Our Perspective on Chronic Disease?
Several decades ago, The People’s Pharmacy interviewed Dr. Paul Cheney, then of Incline Village, Nevada, about his patients with chronic fatigue syndrome. He believed at the time that epidemiological patterns of this mysterious illness pointed to an infectious origin. Years have passed, and no pathogen has been identified to satisfy the criteria as THE cause of myalgic encephalomyelitis (ME/CFS).
Recently, though, millions of Americans have been struggling with a condition that seems rather similar. The only difference is that we know their symptoms began with a COVID-19 infection. Long COVID is difficult to treat. Patients suffering with this condition appear to be afflicted with a serious chronic disease. Researchers have not always found evidence of persistent infection with the SARS-CoV-2 virus. Nonetheless, in most cases a COVID infection was clearly the origin. How has that changed our attitude toward the possibility that infections trigger chronic diseases?
Other Mystery Conditions:
As we contemplate the possibility that infections trigger chronic diseases, we should not overlook chronic Lyme disease. Most infectious disease experts insist it isn’t an infection. Some even resist the idea that people are suffering. Dr. Ewald suggests that perhaps the inability to identify pathogens in the wake of Lyme disease is due to using old techniques.
The pathogens don’t show up on these tests, but that could be because they are hiding. Will newer techniques reveal them? What about the possibility that diseases like arthritis or schizophrenia are caused by pathogens in some cases? The evidence is tantalizing. Dr. Ewald urges us to look at the chronic phases of infection as well as the acute phases.
This Week’s Guest:
Paul Ewald, PhD, is an evolutionary biologist, specializing in the evolutionary ecology of parasitism, evolutionary medicine, agonistic behavior, and pollination biology. He is currently a Professor of Biology at the University of Louisville. Professor Ewald is a pioneer in evolutionary medicine and infectious disease research. He has challenged conventional wisdom on the causes and prevention of many chronic diseases with his idea that many diseases of unknown origin are the result of chronic low-level infections, which has ultimately been shown to be correct for a wide range of diseases to date. He is the author of Evolution of Infectious Disease and Plague Time: The New Germ Theory of Disease.
The People’s Pharmacy is reader supported. When you buy through links in this post, we may earn a small affiliate commission (at no cost to you).

Paul Ewald, PhD, describes how microbes evolve
Listen to the Podcast:
The podcast of this program will be available Monday, Dec. 15, 2025, after broadcast on Dec. 13. You can stream the show from this site (the arrow inside the green circle under the photo at the top of the page) and download the podcast for free. In this week’s extra episode, Joe asks Dr. Ewald how to get specialists to consider the possibility that infections may be at the root of many chronic conditions.
Download the mp3, or listen to the podcast on Apple Podcasts or Spotify.
Transcript of Show 1455:
A transcript of this show was created using automated speech-to-text software (AI-powered transcription), then carefully reviewed and edited for clarity. While we’ve done our best to ensure both readability and accuracy, please keep in mind that some mistakes may remain. If you have any questions regarding the content of this show, we encourage you to review the original audio recording. This transcript is copyrighted material, all rights reserved. No part of this transcript may be reproduced, distributed, or transmitted in any form without prior written permission.
Joe
00:00-00:01
I’m Joe Graedon.
Terry
00:01-00:05
And I’m Terry Graedon. Welcome to this podcast of The People’s Pharmacy.
Joe
00:06-00:27
You can find previous podcasts and more information on a range of health topics at peoplespharmacy.com. Heart disease, diabetes, asthma, Alzheimer’s disease, and arthritis are challenging diseases. Could pathogens be responsible? This is The People’s Pharmacy with Terry and Joe Graedon.
Terry
00:34-00:43
Our guest today, Dr. Paul Ewald, is an evolutionary biologist who’s been studying how pathogens could spark some of our most vexing chronic diseases.
Joe
00:44-00:53
Whether it’s Alzheimer’s disease, rheumatoid arthritis, heart disease, or chronic fatigue syndrome, the cause might be an unsuspected infectious process.
Terry
00:54-01:05
If infections are responsible for a wide range of chronic conditions, treating symptoms might not be effective. How can we treat the cause of many of our most serious and challenging disorders?
Joe
01:06-01:10
Coming up on The People’s Pharmacy, how infections trigger chronic diseases.
Terry
01:14-02:40
In The People’s Pharmacy Health Headlines: Health insurance companies are struggling with their budgets. The enormous popularity of the GLP-1 drugs, such as semaglutide and tirzepatide, is a big part of the reason. These weight loss medications sold under the brand names Wegovy and Zepbound, respectively, are pricey. So the large numbers of people taking them has increased expenses more than expected.
According to stats, some insurers have already spent more in nine months of 2025 than they did in all of 2024. Perhaps as a consequence, some employers are considering leaving these meds off the formulary. Certain states have also dropped them from their Medicaid programs.
Although most states still cover semaglutide for diabetes, North Carolina, California, New Hampshire, and South Carolina are dropping coverage for obesity treatment. In Michigan, Medicaid will cover GLP-1 obesity drugs only for patients who are classified as morbidly obese. Health plans for state workers are also reassessing coverage of these medicines. Some physicians are concerned because people who had lost significant weight are now starting to regain it without their medication. Along with excess weight come additional health risks.
Joe
02:41-03:52
Tattooing dates back thousands of years. Historically, body art served a variety of purposes from religious to healing ceremonies or rites of passage or as an indicator of group identity. In recent years, social media and celebrity influencers have popularized tattoos for millions. But are they safe?
A new study in the Proceedings of the National Academy of Sciences links tattoo ink to inflammation in lymph nodes. The investigator studied the biological reaction to tattoo ink in humans and mice. The dyes that are used accumulate in the lymph nodes and appear to trigger long-term inflammation. The pigments can also be found in the spleen, liver, and kidneys.
This study looked at the impact of tattoo dyes on the immune system. The researchers found that following tattooing, the macrophages were less capable of responding to a number of viruses. The COVID-19 vaccine appears to be less effective for tattooed individuals. The authors call for long-term research into the health effects of tattoos, including the risk of cancer.
Terry
03:52-04:46
There are new data on the benefits of a shingles vaccination against dementia. Shingles is a painful outbreak on the skin of people who had chickenpox earlier in life, often many decades before. The shingles vaccine reduces the likelihood that older people will experience such an outbreak.
Previous studies took advantage of natural experiments in Wales and Australia to determine that the original shingles vaccine, Zostavax, could lower a person’s chance of a dementia diagnosis. Further analysis of these data showed that this vaccination also slows the progression of cognitive impairment in people already living with dementia.
People with dementia who received the shingles vaccine were almost 30% less likely to die from their disease over a nine-year period. People with more advanced dementia appeared to benefit the most.
Joe
04:47-05:23
The flu is back, and it could be an especially challenging season. That’s because the flu virus mutated this year after manufacturers locked in the formula for the vaccine. Canada has seen a dramatic 61 percent increase in flu cases in November.
Now, states such as Colorado, Michigan, and Massachusetts are reporting increased cases and hospitalizations for influenza-like illnesses. If the U.S. follows in the footsteps of countries in the southern hemisphere, such as Australia, New Zealand, and South Africa, we’re likely to see an early and severe flu season.
Terry
05:24-06:17
Intermittent fasting has long been a popular weight loss strategy. Chinese researchers report it also shifts connections between the gut and the brain.
They recruited 25 obese individuals for a two-month study with every other day fasting. Volunteers also provided stool samples at the beginning and end of the study. This regimen resulted in weight loss and also changes in brain activities seen on fMRI. This was correlated to alterations in the gut microbes.
The researchers conclude that intermittent fasting altered the gut microbiome, and that in turn provoked changes in brain regions associated with appetite and addiction.
And that’s the health news from the People’s Pharmacy this week.
Welcome to the People’s Pharmacy. I’m Terry Graedon.
Joe
06:17-06:33
And I’m Joe Graedon. If you ask a cardiologist what causes heart disease, chances are good you’ll hear about LDL cholesterol. Likewise, if you ask a neurologist about Alzheimer’s disease, you’re likely to hear that the culprit is beta-amyloid plaque.
Terry
06:33-06:41
But what if these and many chronic diseases result in part from infections? Would that change the practice of medicine?
Joe
06:42-07:06
To help us answer such questions, we turn to Dr. Paul Ewald, professor of biology at the University of Louisville. He is a pioneer in evolutionary medicine and infectious disease research. Dr. Ewald is the author of “Evolution of Infectious Disease” and “Plague Time: The New Germ Theory of Disease.” Terry was working remotely when we recorded this interview.
Terry
07:08-07:11
Welcome back to The People’s Pharmacy, Dr. Paul Ewald.
Dr. Paul Ewald
07:12-07:14
It’s great to be back to join you again.
Joe
07:15-08:05
Dr. Ewald, I looked back in our calendar and it shows you joining the People’s Pharmacy in April of 1999, show number 263, talking about the evolution of infectious diseases. And then we had you back again in March of 2001, show number 350, “Plague Time: The New Germ Theory of Disease,” which was your second book.
We called that show How Germs Shape Your Destiny. I guess it must be astonishing to you to look back over 25 years and how things have changed. But before you tell us that, please share what is an evolutionary biologist.
Dr. Paul Ewald
08:07-08:34
Well, an evolutionary biologist is someone who just looks at the biological changes of organisms over time. And you can look at it in terms of how they’re adapted to particular environments, or you can do that descriptively, just describing which organisms evolved from what other ones and what characteristics evolved.
My focus tends to be more on the former. I’m interested in how it is that organisms adapt to particular environmental conditions.
Joe
08:35-09:03
So looking back over the last two or three decades, especially with COVID in the mirror, it seems like the kinds of problems that you predicted decades ago have kind of come to pass. Tell us about your view of the world and how pathogens have impacted us since your two books.
Dr. Paul Ewald
09:04-10:21
Well, I would say over the last two decades, the information that’s become available has reinforced the idea that pathogens are pretty much important in almost every aspect of our lives. I was working largely on understanding the causes of chronic diseases.
And over the last two decades, a lot of information has come out that has very gradually indicated that infections are much more important in chronic diseases than we thought. But the way in which they’re important involves interactions between infectious organisms and mutualistic organisms, and also between the genetics of people in the case of human diseases, the genetics of the organisms, and also the non-infectious environmental factors.
So all of these three categories come together, the microbes, the non-microbial environments, things like, you know, do we exercise or do we not? What’s our diet like? And then the genetics, which determines what kinds of things we’re vulnerable to, what kinds of negative things we’re vulnerable to, and what kinds of characteristics we have in place to stay healthy.
Terry
10:22-11:14
Well, it all sounds rather complicated if we have to look at genetics and behavior and environment and pathogens, these infectious organisms. And one of the things that Joe and I have noted is that the infectious disease specialists, the doctors who specialize in treating infectious diseases, they know a lot about antiviral drugs and antibiotics, but they don’t seem that interested in your idea that some of these infectious agents, these pathogens, might be behind chronic diseases like cardiovascular disease or Alzheimer disease. How come?
Dr. Paul Ewald
11:14-12:29
Well, I think that physicians are trained to diagnose and treat. And so we can’t expect that they’re necessarily going to have a focus on this bigger picture of what actually causes disease. They have particular protocols for treating disease once they diagnose them.
And, you know, there’s some pressure on them to do that. If they deviate from the standard protocols, they could be liable for malpractice. And so I think what basically we have to realize is that physicians are trained to do one thing in a clinical setting, diagnose and treat.
And what an evolutionary biologist is interested in doing is trying to understand how all of this fits together. In other words, trying to understand how evolutionary forces shaping humans influence disease, how evolutionary forces shaping microbes influence disease, and how all of that depends on the environments we’re in.
And often that involves noticing that there are mismatches between our current environments and the environments in we evolved and those are the environments in which we generated the adaptations to deal with health and disease.
Joe
12:29-13:50
Dr. Ewald, when we spoke to you two decades ago, I don’t think we had heard of the term microbiome. I mean, everybody knew that there are bacteria and fungi and such organisms in our digestive tract, but microbiome was not a term that was used very much.
Now it seems like everybody’s talking about the microbiome, and it’s not just of the digestive tract. There’s a microbiome of the lungs. There’s a microbiome of the skin. There’s a microbiome of the brain. And the idea that there are pathogens that are living in our bodies, it seems alien to most people, but we’re beginning to gradually recognize, yes, we’re living in quote-unquote harmony or disharmony with a lot of different bugs.
So I’m curious as to how this concept of the microbiome throughout our body is affecting your work in evolutionary biology and the idea that there are a lot of germs, viruses, and bacteria that have set up housekeeping in us and may sometimes cause problems.
Dr. Paul Ewald
13:51-15:47
Well, I think we overlook the microbiome because the members of the microbiome are very small. We don’t see them, okay? So once we recognize that they’re there, then our task is to figure out which of these microorganisms are beneficial to us, actually helping us, and which ones are harmful. And this problem has been a little bit clouded by some of the terminology.
So once microbiome was recognized as being important potentially for our health, then people who are studying this tended to use this term commensal for any organism that wasn’t overtly negative or positive. But in an evolutionary context and in biological context, a commensal is something that neither harms nor helps the host. And so basically, if we really could measure the net effect of all these different organisms, we would classify them all as either parasitic or mutualistic, neither unbalance their net harming us or unbalance their net helping us.
And that seems like sort of an academic distinction, but it’s a really important one because if we’re thinking about supplementing our microbiome, then we want to be supplementing it with mutualists. We don’t want to supplement it with an organism that is slightly pathogenic, especially because sometimes we supplement the microbiome for people who are in particularly vulnerable situations.
And so we’ve learned sort of the hard way that some of the things that look like they’d be good to supplement our microbiome with ended up not being so great, but others ended up being fantastic. And so I think that there’s a bit of a problem in the way in which this has been addressed. But the basic idea is really good, that we’re recognizing that we are not just individuals walking around in an environment. We have our own ecology of organisms in and on us. And we need to understand that if we want to be able to improve health and avoid disease.
Terry
15:49-16:33
Dr. Ewald, I wonder if you could give us an example of one of those microorganisms that we’ve discovered is actually unexpectedly helpful. Sometimes a microorganism that we think is just kind of neutral turns out to be maybe just fine as long as the rest of the microbiome is in balance. But if the microbiome gets out of balance, that neutral guy sitting in there can get out of control. And I’m thinking of Clostridioides difficile, I think.
Dr. Paul Ewald
16:34-17:38
Yes. Well, that is a really great point that we need to be thinking about the effects of the organisms in the context of all the other organisms that are there. And sometimes an organism that is going to be helpful in one context will actually be harmful if the microbiome has changed.
Clostridium difficile is a very interesting example because interest started on this organism about 30 years ago when it was recognized it was causing some problems in hospital settings. And so people found that a lot of individuals are carrying Clostridium difficile without any problem, but they were causing problems in hospital settings.
And so they jumped to the conclusion this organism was a commensal or a very mild pathogen, maybe even a mutualist, without enough data. When you look at Clostridium difficile in a general population, it really doesn’t cause noticeable harm, but that doesn’t mean it doesn’t cause some harm.
Joe
17:38-17:48
Dr. Ewald, we are going to take a break. But when we come back, what we want to do is find out when it causes problems and how to get rid of it.
Terry
17:49-18:05
You are listening to Dr. Paul Ewald. He’s an evolutionary biologist and professor of biology at the University of Louisville. Dr. Ewald is the author of “Evolution of Infectious Disease” and “Plague Time: The New Germ Theory of Disease.”
Joe
18:05-18:09
After the break, we’ll learn how C. diff infections can start to overwhelm hospitals.
Terry
18:10-18:17
Cardiologists pay a lot of attention to cholesterol levels. Should they also keep an eye out for pathogens in the arteries or even the mouth?
Joe
18:18-18:25
We also worry about Alzheimer’s disease. Are there germs that might contribute to its development?
Terry
18:39-18:42
You’re listening to The People’s Pharmacy with Joe and Terry Graedon.
Joe
18:51-18:54
Welcome back to The People’s Pharmacy. I’m Joe Graedon.
Terry
18:54-19:11
And I’m Terry Graedon.
Joe
19:12-19:27
Modern medicine has a tremendous number of specialties and subspecialties. There are not just cardiologists, but interventional cardiologists who perform angioplasty and place stents in coronary arteries.
Terry
19:28-19:37
Neuroimmunologists study multiple sclerosis and neuromyelitis. Such subspecialties may focus very narrowly on a small range of symptoms.
Joe
19:38-19:50
When specialists are stuck in silos, they may not consider the bigger picture. The idea that infections might trigger a number of hard-to-treat chronic diseases is somewhat foreign to them.
Terry
19:50-20:18
We’re speaking with Professor Paul Ewald. He is an evolutionary biologist specializing in evolutionary medicine and pollination biology. He is professor of biology at the University of Louisville. Professor Ewald is a pioneer in evolutionary medicine and infectious disease research. His books include “Evolution of Infectious Disease” and “Plague Time: The New Germ Theory of Disease.”
Joe
20:20-20:29
Dr. Ewald, you were just talking about C. diff infections, and it’s my understanding that they can be really hard to get rid of once they take hold.
Dr. Paul Ewald
20:30-22:03
Yes, and the C. difficile infections are very problematic in hospitals. It used to be thought that they were just causing problems because a person’s microbiome was upset or a person was vulnerable in one way or another because they’re in the hospital.
But when you look at the strains that are in hospitals and the strains in the outside community, you find the strains in hospitals are actually more severe. And this was not recognized for a while. Over the last 10 years, it’s gradually become recognized.
And so what looks like it’s happening is this Clostridium difficile organism is actually evolving increased virulence in hospitals where it can get from one patient to another, even if the patient’s sick. It gets transmitted between patients on the hands of attendants. So it is resistant to antibiotics. Antibiotics are not as effective as we would like them to be.
But there are a lot of ways in which we can deal with C. difficile. And one of the best ways is improving hygiene so that you actually don’t get attendants transmitting the organism from an infected individual to a susceptible individual. And if you do prevent that kind of transmission, you’ll do two things. One, you’ll actually protect individuals who become infected, but also you should actually turn down that evolutionary pressure in the hospital environment favoring the harmful strains.
And so you’ll get a gradual leakage of the milder strains into these hospital environments, and they can protect against the harmful strains through cross-protection immunologically.
Joe
22:04-23:01
Dr. Ewald, I’d like to change gears a little bit now and go back to some of the what were really radical ideas that you were expressing 25 years ago. And let’s just start with heart disease because it is the number one killer in America, if not in the world. And if you were to talk to most cardiologists, they would say, well, the number one killer is caused by cholesterol, in particular, bad LDL cholesterol. And statins are the savior.
And along comes Dr. Ewald and he says, yes, but there are some bacteria that might be responsible and possibly even other pathogens. And I think that’s a hard sell for most specialists in the field of cardiology. So how is it possible that pathogens could be causing heart disease?
Dr. Paul Ewald
23:02-27:38
Well, pathogens invade our blood system, and they can be transported in cells, macrophages, and they can get into the insides of these blood vessels. And when I talked last time, or not last time, but 20 years ago when I was talking with you, I was mentioning some pathogens that had been identified in these lesions, these cardiovascular lesions. One of them is Chlamydia pneumoniae. And there are pathogens from the oral cavity that cause gingivitis and periodontitis that are found there.
And at that point, there were a few studies indicating that there were these associations. People did more studies and some of the studies didn’t agree. And so people sort of lost interest. People tried to treat with antibiotics and the antibiotics weren’t effective in remedying cardiovascular disease. But the microbiologists say, of course, they weren’t.
These microorganisms by that time are living sort of encrusted in all of this decayed tissue. And so the antibiotics aren’t going to get to them.
So the flash forward 20 years, what [has] now been recognized is that with many different studies that are done, mostly outside the United States, because the United States sort of stopped funding this work about 20 years ago. Now, if you look at all those studies together, there’s a very robust trend for chlamydia pneumonia, this respiratory tract pathogen that gets into the vessels of the arteries, the arterial vessels, to be strongly associated with cardiovascular disease.
So people that dismiss that, my response is just look at the literature. The literature has changed so much. It’s become so developed over the last 20 years that now there should be no argument about whether those organisms are there. The only argument is the extent to which they’re actually causing the disease. But there are more data indicating that there’s an answer to that question as well.
And one of the best batches of data has come out of Taiwan, which has this health system where they’re keeping track of everybody’s health records. And what people did in Taiwan was to look to see whether people who came in with Chlamydia pneumoniae pneumonia, that is pneumonia caused by this organism, were, if they were treated, were they less likely to come down, in this case, with Alzheimer’s disease? Because the argument about chlamydia pneumonia applies to Alzheimer’s disease as well as cardiovascular disease.
And so what they found is those individuals that came in with pneumonia caused by Chlamydia pneumoniae, they were treated, did not have an association with Alzheimer’s later on, whereas the ones who came in with chlamydia pneumonia that were not treated did.
Okay, so you’ve got this, what’s getting close to an experiment. You couldn’t run an experiment on people for ethical reasons, but this is pretty darn close. So you’ve got the evidence now for cardiovascular disease and also for Alzheimer’s really being quite overwhelming that this organism’s associated with these diseases.
Now, a similar situation has occurred with the oral pathogens, things like Porphyromonas gingivalis, which is also not only causing periodontal disease, but is associated probably causally with Alzheimer’s disease and with cardiovascular disease.
So going back to the original point about cholesterol and statins, the evidence on cholesterol indicates that, yes, that’s contributing as well. But the actual degree to which cholesterol is contributing looks like it’s modest, but it’s something that’s easy to measure.
And so I think what happened historically is that people measure what they could measure. They can take a blood test. They can easily measure cholesterol and they could find that association. And so they sort of hung a lot of their advice on that association. But just because something’s easy to identify doesn’t mean it’s the main player.
And so when you look at some of these organisms, you find that they actually do better when people have higher fat and cholesterol in their blood. And some of them, like chlamydia and pneumonia, actually increase the amount of cholesterol. So when you find that cholesterol is associated, you have to say, okay, so what’s causing the increase in cholesterol? And you have to reopen the idea that it could be a very complicated set of factors, including microorganisms that are, they are sort of upsetting the system.
Terry
27:38-28:01
Well, Dr. Ewald, you did mention Alzheimer’s disease with reference to Taiwan, where they do have excellent healthcare records. And I think you suggested that people with Chlamydia pneumoniae infections were more prone later to develop Alzheimer disease. Did I get that right?
Dr. Paul Ewald
28:02-28:02
Yeah.
Terry
28:04-28:37
So what I want to ask you about is what we’ve been hearing from the Alzheimer’s disease researchers, not necessarily the ones we’ve been talking to most, but the most prevalent ones, the most prominent ones, is Alzheimer’s disease is caused by buildup of amyloid plaque in the brain. Some of the researchers we’ve been talking to say, yes, but amyloid plaque is actually a response to infection. What’s your take on that?
Dr. Paul Ewald
28:37-29:50
Well, we now know that beta amyloid is a protein that actually is antimicrobial. So if you’ve got infections in the brain, you’re going to have amyloid beta being produced, and that is going to be associated with the degree of threat. So the real problem is thinking about the correlation between the amyloid plaques and the damage to the brain in Alzheimer’s and trying to figure out how much of that is a response to something else and how much of that is actually creating the problem of Alzheimer’s.
And the bottom line, it’s a little bit of both. It looks like the amyloid proteins do have some negative effects, but it is clear that they’re also antimicrobial and they’re elevated. And the particular subsets of amyloid beta are elevated in response to infection and they actually control the infection. So that’s been pretty well looked at for one of these organisms of the oral cavity, periodontal pathogens, in particular, Porphyromonas gingivalis. So it’s been looked at in animal models.
Joe
29:50-30:39
Dr. Ewald, the idea that Alzheimer’s disease or dementia might somehow be precipitated by infection is still pretty radical. And there have been papers about herpes simplex virus as one possible contributor. You’ve now suggested Chlamydia pneumoniae as another possible [contributor]. There may be a whole bunch of infectious agents that are contributing to Alzheimer’s disease.
And I’m just wondering, well, patients want to know, well, what can I do about it? You know, how can I prevent Alzheimer’s disease? How can I prevent heart disease? How can I get rid of those infectious agents that might be contributing to these very serious chronic conditions?
Dr. Paul Ewald
30:41-31:15
Yes, I think you’re exactly right. The emerging trend is that there are a lot of organisms that are involved, including herpes simplex and Porphyromonas gingivalis and Chlamydia pneumoniae. So there are a number of ways in which we can actually prevent this damage.
One way that has been very slow to be assessed, but now it looks like it’s actually having a big effect, is taking better care of your oral cavity. Flossing, for example, looks like it has been associated with a much lower rate of Alzheimer’s. And so…
Joe
31:15-31:26
Whoa, whoa, whoa, wait a minute. Are you telling me that flossing your teeth on a regular basis might reduce your risk of Alzheimer’s disease?
Dr. Paul Ewald
31:26-34:16
That’s what you wanted, Joe. We wanted some practical applications. So let me tell you the mechanism that is almost certainly the right mechanism. When you floss, you take care of your oral health. This could also involve use of antibiotics to control periodontal disease. You’re controlling organisms that are found in the brain and are associated with Alzheimer’s. And you’re also controlling organisms that are found in the artery walls that are associated with atherosclerosis. And you’re also controlling one of the big bad guys I mentioned before, Porphyromonas gingivalis, which contributes to diabetes.
And it looks like that’s a two-way street. Diabetes contributes to porphyromonas growth. Porphyromonas growth contributes to diabetes. And the whole thing is related to these other diseases because diabetes, when it’s bad, is related to bad cardiovascular disease. It’s also related to Alzheimer’s. And almost certainly the mechanism is that when you’ve got high blood sugar, then organisms that are normally sort of kept in check by the immune system are not so easily kept in check.
So these organisms that are contributing to cardiovascular disease and to Alzheimer’s, at least in theory, and probably in practice in reality, they’re not controlled as well by the immune system when you’ve got high blood sugar. And so diabetes then exacerbates these other diseases.
Now, if you ask people, you know, sort of that are not thinking about this in a broad, integrative way, so why is it that people with diabetes have more heart attacks and have more Alzheimer’s and have more periodontal disease? They’ll often say, well, it just messes everything up. Well, this is a very different view. It says that when we understand what the actual causal mechanisms are, we see connections. And that explains why diabetes is so associated with so many of these other chronic illnesses. They’re actually exacerbating the situation by favoring microorganisms that look like they’re involved in the pathology of these chronic diseases.
And so I would just come back to your original point, Joe, and I would just say when people are skeptical, my response is dig deeply into the literature. Look at this information and you’ll see these connections. People are just working in such isolated ways that they’re not seeing these connections. And Terry, as you said, it is complicated. It takes work. And I am sympathetic to physicians, for example, who may not have the time to look at it.
But if you don’t have the time to look at this vast literature that’s emerging, then I would think a little circumspection is in order to say, well, you know, I haven’t looked at the literature. It’s an idea worth considering. Let’s look at the evidence.
Joe
34:16-34:16
Terry?
Terry
34:17-34:52
One thing we do see in the literature in terms of how can we reduce our risk for coming down with Alzheimer’s disease is related to viruses. It turns out that people who are vaccinated against shingles, which is of course caused by the chickenpox virus, are at a significantly reduced risk, not perfectly protected, but significantly reduced risk of developing Alzheimer’s disease or other dementias. You want to comment on that? You know, viruses, they’re pretty important too.
Dr. Paul Ewald
34:53-35:18
Yeah, that was my next point. You beat me to it. I was just going to talk about the varicella zoster virus and how evidence now is really clear, based on a lot of studies, that vaccination against the varicella zoster virus, a shingles vaccination is associated with a quite dramatic decline in the probability of developing Alzheimer’s.
Joe
35:18-35:54
So, Dr. Ewald, it seems like a lot of the specialists, I don’t care whether they’re cardiologists or gastroenterologists, psychiatrists, rheumatologists, they just don’t think about pathogens. They think about blood sugar or they think about cholesterol, but you’re sort of suggesting that they’ve got it backwards, that we need to start looking at the pathogens as the causative agents and everything else is secondary. And you have about 30 seconds to respond before the break.
Dr. Paul Ewald
35:54-36:15
Okay. Well, I think you hit it, the nail on the head. They’re specialists and specialists aren’t thinking about how all these things are connected. But when you look at it, you see that there are these connections, very strong connections, that are generating explanations that really are robust as opposed to explanations that are just dealing with one little part of the problem.
Terry
36:16-36:38
You’re listening to Dr. Paul Ewald. He is a professor of biology at the University of Louisville. Professor Ewald is a pioneer in evolutionary medicine and infectious disease research. He’s the author of Evolution of Infectious Disease and Plague Time, the New Germ Theory of Disease.
Joe
36:39-36:58
After the break, we’ll be talking about some ancient history. When chronic fatigue syndrome first showed up, it seemed to be connected to an infection. Scientists have never identified a single pathogen that’s responsible for this devastating condition. How do they think about it now?
Terry
36:59-37:06
Long COVID has some similarities to chronic fatigue. Is that changing how we understand these problems?
Joe
37:07-37:17
Lyme disease can also cause trouble for a long time, even though tests don’t always show pathogens. Could they be in hiding?
Terry
37:18-37:24
One surprising link is between infection and schizophrenia. What should you know?
Joe
37:24-37:31
Another potential connection is between arthritis and infection. Might it change how we treat joint pain?
Terry
37:39-37:43
You’re listening to The People’s Pharmacy with Joe and Terry Graedon.
Joe
37:52-37:55
Welcome back to The People’s Pharmacy. I’m Joe Graedon.
Terry
37:55-38:12
And I’m Terry Graedon. The People’s Pharmacy is brought to you in part by Spatial Sleep, a non-drug approach to help you fall asleep and stay asleep without medications. More information at SpatialSleep, S-P-A-T-I-A-L, sleep.com.
Joe
38:13-38:23
When Dr. Paul Chaney described the first outbreak of chronic fatigue syndrome, he suggested an infectious origin. His colleagues were skeptical.
Terry
38:24-38:42
Our guest today, Dr. Paul Ewald, proposes that many chronic conditions could be rooted in infections. He is professor of biology at the University of Louisville and author of Evolution of Infectious Disease and Plague Time, the New Germ Theory of Disease.
Joe
38:44-39:57
Dr. Ewald, many decades ago, even before we spoke with you, we talked with Dr. Paul Cheney, who was, I think, an internist in Nevada. And he saw a bunch of people who had come down with a rather odd condition where they had terrible fatigue and couldn’t think very clearly after they came down with an infection of some sort.
And he basically was the first clinician, as far as I can tell, who identified what we now call chronic fatigue syndrome or ME/CFS, as some people refer to it. And that idea that you could have this rather nasty upper respiratory tract infection, kind of like the flu, but it never completely goes away. And you’re kind of left with, you know, exhaustion on exercise and brain fog and a whole bunch of other symptoms.
And that seems a little reminiscent of long COVID. How has COVID changed the way we think about these kinds of problems?
Dr. Paul Ewald
39:57-44:07
Well, I would first say that the idea of looking for infectious causes of chronic fatigue syndrome makes a tremendous amount of sense because we know that when infections occur, one of the things the brain does is makes us feel fatigued. And so if you have a persistent infection, you’re likely to feel fatigued for a longer period of time, depending on how persistent it is.
Now, if we flash forward to SARS-CoV-2, and what has become apparent is that the acute phase is part of it, and then there’s a long chronic phase, and people disagree about whether the organism’s still there. I suspect it still is, in refugia–it’s hard to find out whether it’s there or not, if it’s there in very low densities. I would, in answer [to] your question, what has COVID told us about or informed us about, I would say it’s informed us about a lot, but not enough.
Okay. I think there are a lot more lessons. And one of the lessons is that we need to be thinking about infectious diseases much more in the context of both acute and chronic phases, because the acute phase is just part of the story. As soon as you start looking at a chronic phase, people will start saying, oh, well, we don’t see the organism.
Well, the organism’s not as abundant in the chronic phase if it’s there. Also [it] may be causing problems much more indirectly. And so we have the same kind of problem with Lyme disease, where people are arguing that a lot of these chronic correlates of Lyme disease are not because the organism’s still there because their tests don’t show it. Well, again and again, over the last few decades, we’ve found that people are dismissive of infectious causes because they’re using the old techniques that are not sensitive enough, when you start using new techniques and you start thinking more broadly about the ways in which disease organisms can be causing chronic disease, then things appear that you didn’t think were there. So I would argue that for COVID, we need to really be focusing on thinking about detecting pathogens, the virus that could be there in the long run, and then thinking about how we would combat that.
The other lesson from COVID is one that I think we may have talked about the last time I was talking with you, which is that evolutionary thinking informs us that organisms like the coronavirus that causes COVID, those viruses are dependent on hosts being not healthy, but not terribly sick for transmission because they’re moderately durable in the external environment. And the evolutionary theory, which is really supported by a comprehensive evaluation of all human diseases, indicate that if a pathogen is really durable, it’s likely to evolve to be very harmful. If it’s very non-durable in the external environment, it’s likely to be mild. And if it’s in between, it’ll evolve to be in between.
And so one of the points I was making back in 2020 was that we can expect that SARS-CoV-2 is going to be evolving towards a level of virulence that is very much like influenza because that’s how durable is the external environment. And unlike what a lot of people, most people would argue that, oh, it could just become virulent again with a new mutation, I would argue that it will not become more virulent with new mutations over the broad population because those variants will be too harmful for the mode of transmission of this virus. And so that’s a test we can look at.
I made that prediction 2020. So far, it’s held up. The organism over about a year evolved to be more mild and it has not evolved to be more severe like the earlier strains were. And so it’s a prediction from evolutionary thinking that we will be able to evaluate as time goes on. And hopefully people will look back and see that the evolutionary perspective generated these predictions. And if the predictions don’t hold up, then we can say the evolutionary perspective is not great. But if they do hold up, then it lends credibility to this evolutionary perspective.
Joe
44:07-44:10
Well, we certainly hope you’re right. Terry, you have another question?
Terry
44:10-44:35
I do. I’m wondering, Dr. Ewald, you say that we’re using old techniques, old technology, presumably, to look for these pathogens that have caused an infection, and we assume the person is now recovered, and yet they still are feeling bad. The tests that we use don’t show that the pathogen is there. Could a pathogen be hiding?
Dr. Paul Ewald
44:35-48:40
Yes. Well, I think that’s exactly why they’re hard to detect. They’re essentially hiding. They’re in places where the immune system can’t get to them, and so it’s harder for us to identify them because it’s harder for us to get to those places. [They] may not be as abundant in the body and they also might be much more hidden.
So if the immune system can’t get to them, that’s why they’re persisting. We may not have an antibody response that’s very high. And so people say, well, there’s a slight antibody response, but it doesn’t really look like an active infection, but it’s very well likely to be a moderate antibody response. This is associated with, like you say, a hiding infection.
And this is really quite important because what it means is we have to be able to generate tools that will identify pathogens that are there in much lower density and in tissues where they’re not so obvious. And this is very apparent in cancer, for example, because it used to be thought that if a pathogen was causing a cancer, you would see it in essentially all cells in the tumor, right? And it makes sense. And the first cancers that were accepted as caused by infection did have pathogens that were present in virtually all cells. And so people then presume that that would be the model for all viral-induced cancers.
But now we know that some cancers are caused by viruses that are only present with about 1% of the cells in the tumor. So Hodgkin’s lymphoma is an example of that. And so what that means is we have to be looking much more carefully at all of those cells. And there are techniques now: you can do techniques that involve looking at single cells and then putting all of those cells together, let’s say in a tumor, to see what the overall structure is. And then you can assess whether just a few of those cells are actually cancer cells. And other cells might be infiltrating cells. There might be cells that have lost a virus and therefore are not infected anymore.
So I think that this is a really important issue. People have rejected the idea that infections are causing cancers because they’re found in, let’s say, only 1% of the cells. But now we know that cancers can be caused by viruses that are only affecting 1% of the cells. In the case of Hodgkin’s lymphoma, where this has been accepted, it was a little more obvious because those cancer cells look different. Okay.
And so people [say], what are those cells doing? They found out that those cells were the ones who were infected with the Epstein-Barr virus. Other cells in the tumor were not, and those were the cells that are cancerous. Okay. So you have a clue, it’s kind of conspicuousness of infectious causation. And what we have to remember is we’ll identify and accept infectious causation for diseases in which the infectious causation is more conspicuous than it is in other diseases that are caused by infection, right? Because we will, if it’s conspicuously caused by infection, then everybody can agree on it faster. If it’s inconspicuously caused by infection, then people are going to argue about it.
And so that actually has been the history of the germ theory for the last 130 years, is that we’ve identified the infectious agents that are conspicuously causing infection. And then we’ve argued about the ones that are less conspicuously caused, and then we solve those. And then we argue about the other ones because they’re even less conspicuously caused.
And so now we’re arguing about things like cancer in which you have only a few cells that may be infected in a tumor, a few cancerous cells in the tumor. And we’re dealing with cancers like breast cancer, for which there are six different viruses that have been rigorously associated with breast cancer. This is with multiple analyses and looking at the various studies and using meta-analyses to see what the overall trend is. And so if you’re looking to see whether one virus is associated with breast cancer, it might not be in that population, but another virus might. You have to be thinking about all five, I’m saying all six viruses that have been significantly associated with breast cancer and probably more that haven’t yet been associated.
Joe
48:40-48:42
Dr. Ewald, we are running out of time.
Dr. Paul Ewald
48:42-48:42
Okay.
Joe
48:43-49:47
And I’d like to ask you about schizophrenia.
Dr. Paul Ewald
48:47-48:47
Yes.
Joe
48:47-49:41
Because when you mentioned that a couple of decades ago, I think it came as a real shock to our listeners. How could mental illness, something severe like schizophrenia, be caused by a pathogen?
And just in the last several months, there’s a story in the popular media of a woman who was diagnosed with schizophrenia for many, many years. And then she came down with something that required an antibiotic. And after a course of treatment for whatever infection she had, all of a sudden, her schizophrenia disappeared for good. And it was like, how could that possibly happen?
And so can you give us, in a short period of time, your overview of schizophrenia in particular and how there might be an infectious cause?
Dr. Paul Ewald
49:43-51:43
Okay. So schizophrenia is a great example of a disease entity that’s an umbrella category. And that category used to be embedded in an even bigger category, which included syphilitic insanity. And your question was, how could a pathogen cause such severe mental illness? Well, syphilis, the syphilis organism does it. It was recognized. And as soon as they recognized it, they separated it off from what we now call schizophrenia.
And so for the last hundred years, we’ve been dealing with this term schizophrenia. And I think we’re poised on the edge of making some more divisions, taking away what we’re calling schizophrenia and putting it in another category. So one big advance was to recognize that a lot of schizophrenia really has mood associations. And so in the last 10 years, there’s been a tendency to talk about schizoaffective disorder.
And when we look at pathogens, one thing we find is now with many studies, there’s a highly significant association between Toxoplasma gondii, this cat-rat pathogen, and schizophrenia. But in particular, it seems to be associated with schizoaffective disorder. So I think what we’re poised on doing now is looking at schizophrenia and saying, we want to take off certain parts, carve out certain parts of what we’re calling schizophrenia, and we’ll put it into, make a new category, and then we’ll be left with a smaller category.
And this has been happening, as I said, for over 100 years for psychoses. And so what we can imagine is a new category that we can call ‘toxoplasmal schizoaffective disorder,’ which will be maybe as much as a third of what we’ve called schizophrenia out and put it into this new category.
Then we’ll be left with two thirds of something we don’t understand very well. And we have to look carefully at it and figure out whether there are other subsets that we can carve out in a more realistic category that represents an understanding of the causation of those problems.
Joe
51:45-52:00
Dr. Ewald, we only have two minutes left, could you quickly squeeze in something about arthritis, especially rheumatoid arthritis, and then sum up what people should learn from your books and from your research?
Dr. Paul Ewald
52:02-54:50
Well, arthritis is, again, a big umbrella category. We’ve recognized that some arthritis is caused by infection. And when we recognize it, we carve off that aspect of arthritis and give it a new name. So we’ve given some arthritis a new name, reactive arthritis, which indicates that it is associated with and caused by infection with, in this case, bacteria. And particularly infection with Chlamydia trachomatis, a sexually transmitted pathogen also associated with Neisseria gonorrhoeae.
And so that’s an example of what has happened in this process in which we take these umbrella categories and subdivide off. I think we’ll see more of that kind of subdivision. In the case of rheumatoid arthritis, we know that this is an antibody-mediated disease. The antibody is causing a lot of problems. So what is causing the antibodies to misbehave? Okay. We don’t expect the immune system just to misbehave on its own. Something’s got to be pushing it.
And so there are pathogens that look like they’re associated with rheumatoid arthritis, and we need to really look at them. So Epstein-Barr virus, one that is associated with Hodgkin’s lymphoma, looks like it’s associated with rheumatoid arthritis. Also, the one I mentioned before, the periodontal pathogen, Porphyromonas gingivalis, looks like it’s associated. And the details really look like those associations are causal. So I think it comes back to what can you do to reduce the chance of having these infections? And the Porphyromonas [gingivalis] comes back to flossing, weirdly. How would you ever expect that flossing would be related to protecting yourself against rheumatoid arthritis? But it also raises a general question, which is really important now in this atmosphere of our politics, our governments, and our social setting. And that is that there’s this tendency among some people to think that vaccines aren’t extraordinary tools that have helped the medical sciences to combat diseases. And I think, again, looking at the evidence, you have to realize it’s one of the great categories of advancement.
And it’s likely to be even greater in the future as we recognize a lot of these pathogens we don’t have vaccines for are causing chronic diseases. And some of the pathogens that we have vaccines for are causing more problems than we thought they were causing. So I think that a shout out to the idea that we really have to be thinking clearly about the value of vaccines. Vaccines do have some side effects, but the side effects are so rare compared to the benefits that I think we really should be hesitant to act against the administration of vaccines and also the support for vaccine research.
Joe
54:52-56:06
Dr. Ewald, you have described a whole bunch of chronic conditions that could be triggered by pathogens, by bacteria, viruses, perhaps some other organisms, whether it’s cancer or whether it’s schizophrenia or whether it’s heart disease. And it feels like we’ve just scratched the surface.
If you could pull together all of the specialists, the cardiologists, the pulmonologists, the psychiatrists, the gastroenterologists, and put them in a room and say, hey, guys, hey, women, all of you professionals, you need to start looking at the causes of the conditions that you’ve been diagnosing and treating for decades. And some of those causes, many of those causes, may be pathogens. And until you start killing off or preventing those pathogens from causing the diseases that you’re treating, you’re fighting a losing battle. How could you ever accomplish that huge feat?
Dr. Paul Ewald
56:10-58:11
I have been trying to work towards that end by sort of continuing to write on these issues, continuing to show how certain explanations are missing certain things and how those missing parts are filled in. And you look at interconnections between genes, environment, and infection.
And so I would just say that this is nothing. This slowness is nothing new. It’s been happening for over 100 years. And we just have to have patience. And I don’t think that getting everybody in the room is going to do it. I think we’ve got to actually have papers written, books written, that actually people can take time to read and ponder.
And then people who tend to be leaders in these areas will say, hey, wait a minute, I think we have been a little bit wrong. And then the people that tend to be followers will say, well, this leader said that we’ve been wrong in neglecting this interface between genetics and infection and environment.
And so maybe it makes sense. And so then the default, as people shift, is to then give some credibility to these arguments. But, you know, progress happens. It’s just very slow. It’s like slow motion germ theory of disease.
You know, the germ theory started millennia ago, actually, but certainly centuries ago. So and then the progress has been very slow. And the slow progress has been because the things that are to be discovered in the future are less obviously caused by infection. We just have to get people to realize that.
And I think, I’m thinking the best way is by writing books and papers that people can read, take their time with and ponder rather than trying to get people in a room and sort of make arguments based on evidence that then goes by so fast. And the meeting would go by so fast that people then leave and they’re not changed by it. That’s my sense. And also, I think it’s good to have shows like your show where we can actually get these ideas out.
Terry
58:12-58:18
Dr. Paul Ewald, thank you so much for talking with us on The People’s Pharmacy today.
Dr. Paul Ewald
58:19-58:20
Thank you for having me. It’s been a pleasure.
Joe
58:22-58:48
You’ve been listening to Dr. Paul Ewald, professor of biology at the University of Louisville. He’s a pioneer in evolutionary medicine and infectious disease research. Professor Ewald has challenged conventional wisdom on the causes and prevention of many chronic diseases. He’s the author of “Evolution of Infectious Disease,” and “Plague Time: The New Germ Theory of Disease.”
Terry
58:49-58:57
Lyn Siegel produced today’s show. Al Wodarski engineered. Dave Graedon edits our interviews. B.J. Leiderman composed our theme music.
Joe
58:58-59:05
This show is a co-production of North Carolina Public Radio, WUNC, with the People’s Pharmacy.
Terry
59:06-59:22
Today’s show is number 1,455. You can find it online at peoplespharmacy.com. That’s where you can share your comments about this episode. You can also reach us through email, radio at peoplespharmacy.com.
Joe
59:23-59:39
Our interviews are available through your favorite podcast provider. You’ll find the podcast on our website on Monday morning. The podcast this week has additional information on how to consider the possibility that many chronic diseases are caused by pathogens.
Terry
59:40-01:00:10
At peoplespharmacy.com, you could sign up for our free online newsletter. And that way, you can get the latest news about important health stories. When you subscribe, you also get regular access to information about the weekly podcast. We’d be grateful if you’d consider writing a review of The People’s Pharmacy and posting it to the podcast platform you prefer. If you find our topics interesting, please share them with friends and family.
Joe
01:00:11-01:00:14
In Durham, North Carolina, I’m Joe Graedon.
Terry
01:00:14-01:00:49
And I’m Terry Graedon. Thank you for listening. Please do join us again next week. Thank you for listening to the People’s Pharmacy Podcast. It’s an honor and a pleasure to bring you our award-winning program week in and week out. But producing and distributing this show as a free podcast takes time and costs money.
Joe
01:00:50-01:00:59
If you like what we do and you’d like to help us continue to produce high-quality, independent healthcare journalism, please consider chipping in.
Terry
01:01:00-01:01:04
All you have to do is go to peoplespharmacy.com/donate.
Joe
01:01:05-01:01:18
Whether it’s just one time or a monthly donation, you can be part of the team that makes this show possible. Thank you for your continued loyalty and support. We couldn’t make our show without you.
Citations
- Huang X et al, "Porphyromonas gingivalis aggravates atherosclerotic plaque instability by promoting lipid-laden macrophage necroptosis." Signal Transduction and Targeted Therapy, May 23, 2025. DOI: 10.1038/s41392-025-02251-6

