Proton Pump Inhibitors (PPIs) are extremely popular. That’s partly because they have been widely promoted. Plus doctors routinely prescribe these acid-suppressing drugs because they are great at healing ulcers. The FDA considers this category of medicines so safe it decided to allow the sale of PPIs for heartburn without a doctor’s supervision.
Prilosec OTC (omeprazole) got a green light in 2003. That was followed by lansoprazole (Prevacid 24HR) and esomeprazole (Nexium 24HR).
Are proton pump inhibitors being overused? We worry about the widespread use of PPIs for heartburn. Should drugs with so many potential side effects continue to be sold over the counter?
Do People Read OTC Drug Labels?
I wonder if people taking PPIs for heartburn pay attention to the instructions.
Here is what you will find on the label of Prilosec OTC:
• “Do not use for more than 14 days unless directed by your doctor.
• “You may repeat a 14-day course every 4 months.
• “Do not take for more than 14 days or more often than every 4 months unless directed by a doctor.”
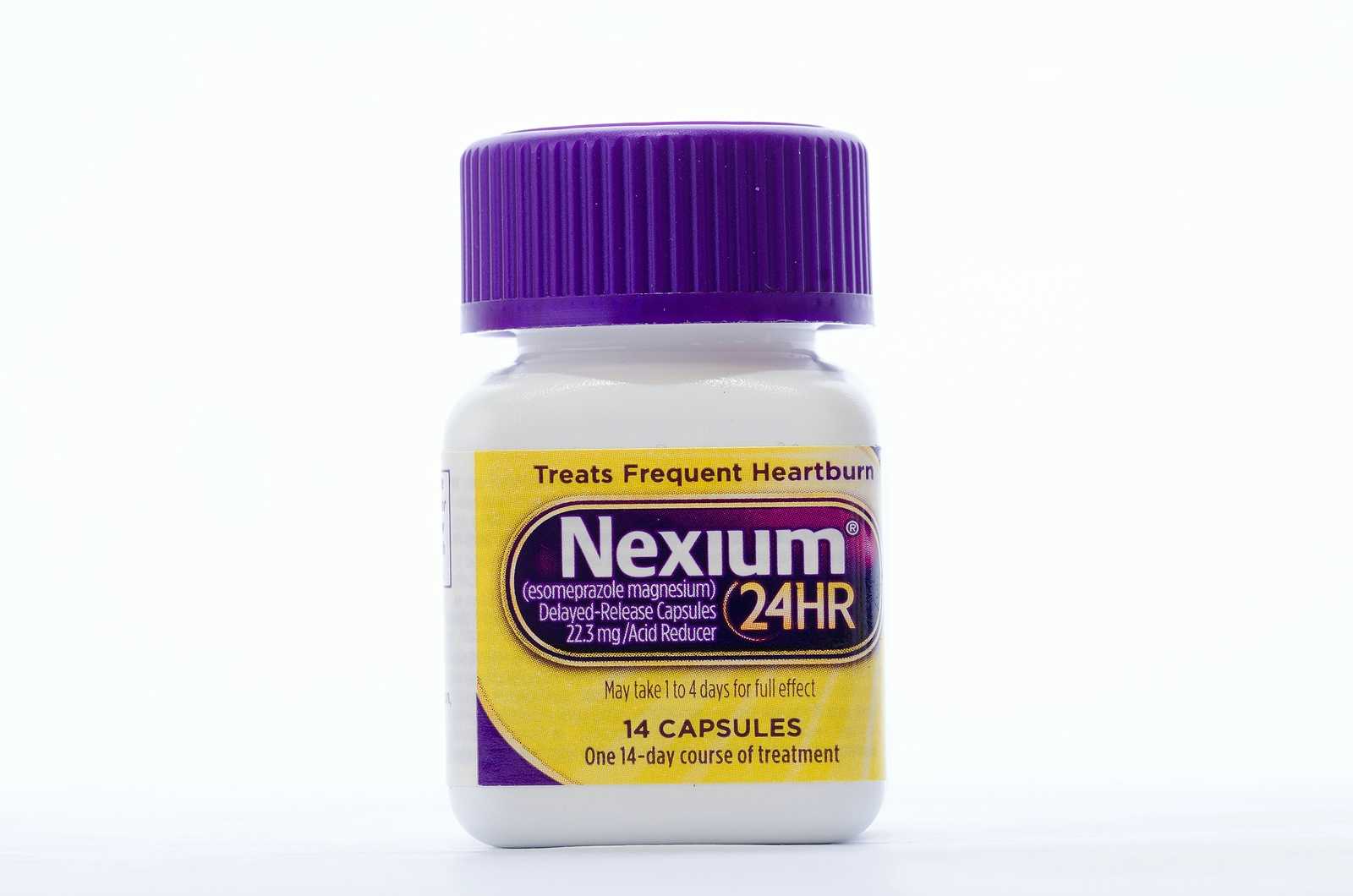
Here are the directions for Nexium 24HR:
• “Do not use for more than 14 days unless directed by your doctor.
Repeated 14-Day Courses (If needed)
• “You may repeat a 14-day course every 4 months.
• “Do not take for more than 14 days or more often than every 4 months unless directed by a doctor.”
Do consumers take the time to read this kind of information? A study of nonprescription drug labels in the US reports the following (Pharmacy, Dec. 2018):
“A well-designed DFL [Drug Facts Label] will be ineffective if not read by consumers. Unfortunately, multiple studies suggest that less than half of consumers read the entire package labeling before taking OTC medicine. For example, only 48% of subjects stated that they always read the usage instructions for OTC pain relievers before use. Similarly, another study found that only 42% of subjects said they read everything on the label when taking an OTC medication for the first time and only 26% report reading the active ingredients at first use. Eye-tracking studies also suggest consumers spend less time viewing warnings compared to other aspects of package labeling (e.g., the brand name). Indeed, brand names may bias some consumers against comprehensive review of the DFL.”
PPIs for Heartburn Can Cause Rebound Hyperacidity:
Do consumers understand what they read and follow the directions? I wish there were better research to answer that question. One study offers these disappointing results (American Journal of The Medicine Sciences, Nov. 2016):
“NSAID misuse was common, with 19% using more than the recommended dose and 24% using multiple NSAIDs concomitantly.”
That does not give us confidence that users of PPIs follow the directions to only take such drugs for two weeks at a time. If someone wants to “repeat a 14-day course” they have to wait 4 months! What if you have heartburn during that 4-month “rest” cycle? Do you really think people are likely to skip taking PPIs for heartburn?
There are a growing list of side effects associated with PPIs. Occasional use doesn’t seem terribly worrisome, but many people take these powerful acid-suppressing drugs for a lot longer than 14 days. And once someone takes a PPI for more than several weeks it can be hard to stop. Here is a link to learn more about this Catch-22.
How Can You Get Off a PPI Without Withdrawal?
Stomach Cancer and PPIs:
Researchers have raised the alarm that powerful acid-suppressing drugs might increase the risk for stomach cancer (Gut, Jan. 2022). In comparison to cimetidine or famotidine users, those taking PPIs were 45 percent more likely to develop stomach cancer during the five-year follow-up period. In the UK where the data were collected, stomach cancer is quite rare, so even a big increase in risk did not result in a large number of people developing this cancer.
This is not the first signal that PPIs might be linked to stomach cancer, though. A systematic review involving 13 studies and more than one million volunteers concluded that PPI users were at double the risk of developing gastric cancer (Therapeutic Advances in Gastroenterology, Nov. 10, 2021). You can read our overview on this issue at this link.
What About PPIs and COVID-19?
A provocative study published in the American Journal of Gastroenterology (Oct. 2020) was titled: “Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors.” The authors conclude:
“In a nationwide study of individuals with a history of GI symptoms, we found that use of PPIs is associated with increased odds for reporting a positive COVID-19 test. The highest risk is seen among individuals taking PPIs twice daily—a common off-label practice in both primary and secondary care—because they are nearly 4-times more likely to report COVID-19 positivity when compared with those not on PPIs.”
The authors are quick to note that this study did not prove cause and effects, but:
“In the meantime, our findings support good clinical practice that PPIs should only be used when indicated at the lowest effective dose, such as the approved once-daily label dosage of over-the-counter and prescription PPIs. Additional studies should also assess whether there is an association between PPIs and indicators of COVID-19 severity, such as hospitalization, need for intubation, or mortality.”
How Could PPIs for Heartburn Be Linked to COVID-19?
The authors of the study also point out that:
“Proton pump inhibitors (PPIs) increase the risk for enteric [intestinal] infections which is likely related to PPI-induced hypochlorhydria [low stomach acid].”
The stomach is normally a hostile environment for bacteria and other nasty pathogens. That includes coronaviruses. The predecessor to SARS-CoV-2 was SARS-CoV-1, also known as just plain SARS. When the pH of the stomach is equal to or less than 3, the “infectivity” of the virus is impaired. In other words, when there is normal acid in the stomach, viruses have a hard time surviving and multiplying.
By suppressing stomach acid, PPIs could conceivably make it easier for SARS-CoV-2 to set up housekeeping in the intestinal tract. That could lead to inflammation and the spread of the virus to other places in the body, including the lungs.
The authors did not discover a relationship between use of other heartburn medicines such as H2 antagonists like cimetidine (Tagamet) or famotidine (Pepcid) and COVID-19.
What to Do About PPIs for Heartburn?
We would be the first to admit that any relationship between PPIs and vulnerability to COVID-19 is speculative at this time, despite biological plausibility. We will need much more rigorous research before we will have a definitive answer about a possible connection between proton pump inhibitors and the novel coronavirus.
There are other considerations, however. Over the last several years we have observed a surprisingly large number of potentially serious complications associated with PPIs for heartburn. They include:
- C. difficile intestinal infections
- Pneumonia
- Kidney stones and kidney damage
- Cardiovascular complications
- Dementia
- Bone fractures
- Liver damage
- Thyroid problems
- Premature death
- Weakened bones/osteoporosis
A Reader Shares Her Story:
The trouble with such a long list of side effects is that people’s eyes glaze over before they get to the end. Some of our readers have shared their personal experiences with PPIs.
Here is one example:
“Approximately 30 years ago, I had severe gastritis for which the doctor prescribed Prilosec. This was a new drug available only by prescription at that time. At first, I heard that I could only use it for a maximum of three months because of possible adverse effects of gastric cancer.
“I stayed on it for about a year because it was very effective against my ulcer. Finally I stopped taking it.
“Approximately 25 years later, I had a terrible chronic cough, which was misdiagnosed as anything from allergies to possible pulmonary, ENT, GI, or cardiac issues. Eventually I was diagnosed with atypical GERD or ‘non-acidic reflux’ and was put on very high-dose PPI treatment.
“After a few years on the PPI, I developed kidney stones. That was really horrible and landed me in an ER. What’s more, my bone density dropped significantly.
“No one saw any connection between my PPI use and these problems. In fact, they blew me off when I asked. I then just weaned myself off the PPI, using OTC Zantac for the pain. I lost weight too, which undoubtedly helped. So did smaller meals.
“I also left that primary care doc who refused to consider PPI as contributing to my sudden drop in bone density or kidney stones!”
We are starting to wonder whether the FDA should reconsider the OTC use of PPIs for heartburn when there are so many other options that seem much safer. When a physician is prescribing PPIs for stomach ulcers, we do not have a problem. That’s because the doctor can monitor progress and help the patient discontinue the PPI when it is no longer necessary.
When people can take PPIs for heartburn without any medical supervision, it makes us nervous. People taking such drugs without a prescription must pay attention to the warning limiting use to two weeks at a time. There should be a 4-month break between treatments.
Final Words:
If you would like to learn much more about the pros and cons of PPIs for heartburn you may wish to read our newly revised and expanded eGuide to Overcoming Digestive Disorders. This electronic resource is available in the Health eGuide section of this website. You will also learn about alternatives to PPIs for heartburn. For example, famotidine (Pepcid) may actually help improve outcomes if patients develop COVID-19 (Gut, June 4, 2020).
Although this was a small, 10-patient case series, the authors conclude:
“The results of this case series suggest that high-dose oral famotidine is well tolerated and associated with improved patient-reported outcomes in non-hospitalised patients with COVID-19.”
This is not the first report of famotidine vs. COVID-19.
A study published in the journal Gastroenterology (online, May 22, 2020) concluded:
“This retrospective study found that, in patients hospitalized with COVID-19, famotidine use was associated with a reduced risk of clinical deterioration leading to intubation or death…The results were specific for famotidine (no protective association was seen for PPIs) and also specific for COVID-19 (no protective association in patients without COVID-19).”
The People’s Pharmacy Perspective:
Most people think that over-the-counter PPIs for heartburn are perfectly safe. The report suggesting that there may be an association between PPI use and susceptibility to COVID-19 is worrisome. But it needs corroboration. There are lots of other serious side effects to consider.
The FDA insists that it only approves drugs that are “safe and effective.” If you scroll back up to the possible complications associated with PPIs for heartburn, it is hard to say that this class of medicines is without complications.
No one should ever stop taking a PPI suddenly. Rebound hyperacidity and severe heartburn symptoms could result. If you are under a physician’s care, ask your doctor about this study in the highly regarded American Journal of Gastroenterology. If you are taking PPIs for heartburn, you may want to consult our eGuide to Overcoming Digestive Disorders to learn about other options.
Please share your thoughts about PPIs and other treatments for indigestion in the comment section below. If you think this article might be of interest to a family member or a friend, please share it by scrolling to the top of the page and using one of the icons for email, Facebook or Twitter. Thank you for supporting our work in this manner.
Citations
- Almario, CV et al, "Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors." American Journal of Gastroenterology, Preprint, online, July 7, 2020.

