Most people take prescription medications to relieve symptoms or prevent serious health problems. Some of the best sellers include blood pressure medicines, acid-suppressors, diabetes drugs and medications to ease pain and inflammation. I am talking about drugs such as lisinopril, losartan, metformin and omeprazole. People often take such medicines for years or even decades. They do not imagine that a medicine that is supposed to improve their health might pose a cancer risk. Even some body care products such as antiperspirants or deodorants have been found to contain benzene, a known carcinogen.
The Dreaded
People used to worry a lot about heart attacks. No surprises there. Cardiovascular disease has been the big killer of Americans for decades. But quick intervention with stents can save lives. A dear friend had a “myocardial infarction” while hiking. Quick action by a team of interventional cardiologists opened the clogged coronary artery and had him home within a day or two. His prognosis is excellent.
The same cannot be said about cancer. Ask people what disease they fear most, and you are likely to hear about malignancy. A diagnosis of breast cancer, prostate cancer, lung cancer, glioblastoma or lymphoma is terribly traumatic. Such a diagnosis of cancer impacts your life like a sledge hammer. Anxiety, anger, fear and depression are just a few of the emotions that can turn your world upside down. For many cancer patients, life is never the same.
Avoiding such malignancies is always best, but prevention is challenging. People are told to avoid tobacco products because smokers run a higher risk of many different kinds of cancer. Tobacco smoke contains numerous carcinogens, including benzene and nitrosamines.
A Cancer Risk from Medicines and Body Care Products?
What comes as a shock to many health professionals as well as consumers is the presence of such carcinogens in some very popular medicines and body care products. The pharmaceutical industry and the Food and Drug Administration haven’t known what to do about this problem.
Even though animal research that the FDA sometimes requires may reveal a risk of tumors, that alone doesn’t keep a drug off the market. Long-term follow-up studies in humans are relatively rare. As a result, patients have no way of knowing whether the medicine they swallow every day could be increasing their risk of cancer later in life.
The same thing is true for some body care products. That includes hand sanitizers, sunscreens, deodorants and antiperspirants. Most people have a hard time imagining that a product they use to spritz their pits or spray on their child might have carcinogenic contaminants.
Cancer Risk and Blood Pressure Meds?
One of the most controversial examples involves the popular blood pressure pills called angiotensin receptor blockers (ARBs). You might recognize irbesartan (Avapro), losartan (Cozaar), telmisartan (Micardis) and valsartan (Diovan), among others. These are very popular BP medications. That’s partly because they are less likely than ACE inhibitors such as lisinopril to cause a cough.
The debate goes back more than a decade. In 2010, a meta-analysis suggested a link between ARB use and lung cancer (Lancet Oncology, July 2010). The FDA reviewed this potential risk itself.
A year later, the agency declared:
“The U.S. Food and Drug Administration (FDA) has completed a review of the potential risk of cancer associated with the class of medications known as angiotensin receptor blockers (ARBs). FDA has concluded that treatment with an ARB medication does not increase a patient’s risk of developing cancer.”
There was no hedging and no doubting. The FDA declared “all clear.” Relax! Take your pills and do not worry.
Timing Is Everything When It Comes to Cancer Risk:
At the time, we were surprised to discover that most of the trials FDA had reviewed were relatively short term. It can take years for cancer to develop, so short-term studies may not be able to reveal such a risk. In fact, experts at the National Toxicology Program told us they doubted you could even detect a lung cancer risk from smoking in just three years or so.
This spring, a scientist published an analysis that throws the FDA’s assurances into question (PLOS One, March 2, 2022). His review covered 15 randomized controlled trials with 135,218 patients.
He found that cumulative exposure made a difference. In other words, people taking high doses over more than three years had an increased risk of cancer. In particular, a higher chance of lung cancer kicked in after just 2.5 years.
He added:
“In conclusion, this analysis, for the first time, reveals that risk of cancer with ARBs (and specifically lung cancer) increases with increasing cumulative exposure to these drugs. The excess risk of cancer with long-term ARB use has public health implications.”
Over the last several years, nitrosamines have been detected in a number of different ARBs. Whether this contamination is contributing to a cancer risk is hard to say.
The FDA has offered this reassurance and explanation:
“ARBs, including valsartan, irbesartan, losartan and others, are a class of medicines used to treat high blood pressure and heart failure. Nitrosamine impurities, including N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA), are probable human carcinogens. These two substances are known environmental contaminants and found in water and foods, including meats, dairy products and vegetables. But their presence in drug products is not acceptable.
“…Our analysis of NDMA found that the risk to patients based on the maximum possible exposure appears to be small. That doesn’t diminish our concern and our determination to find out how these impurities occurred in the first instance.
“…While we’re still investigating the root causes of the impurities, our ongoing effort has determined that the impurities may be generated when specific chemicals and reaction conditions are present in the manufacturing process of the drug’s API, and may also result from the reuse of materials, such as solvents.”
FDA’s Response to the Zantac (Ranitidine) Cancer Risk:
In our view, the FDA seems to take a somewhat cavalier attitude about the cancer-causing potential of many medications. Take the popular heartburn medicine ranitidine (Zantac). An independent laboratory found the probable carcinogen NDMA in various samples of this drug.
Many other countries recalled this medication from pharmacy shelves.
The FDA followed with an announcement that there is a:
“voluntary recall of over-the-counter (OTC) ranitidine tablets (75 mg and 150 mg), labeled by Walgreens, Walmart, and Rite-Aid and manufactured by Apotex Corp. These medicines may contain low levels of a nitrosamine impurity called N-nitrosodimethylamine (NDMA).”
The agency also announced a voluntary recall of prescription-strength ranitidine made by Sandoz.
On September 13, 2019 the FDA reassured the American public that:
“Patients should be able to trust that their medicines are as safe as they can be and that the benefits of taking them outweigh any risk to their health. Although NDMA may cause harm in large amounts, the levels the FDA is finding in ranitidine from preliminary tests barely exceed amounts you might expect to find in common foods.”
The Food and Drug Administration went on to state:
“FDA is not recommending individuals stop taking all ranitidine medicines at this time.”
Valisure, the pharmacy that detected NDMA in ranitidine, asked the agency to:
“recall and suspend sale of all lots of all products containing ranitidine.”
It maintains that levels of the nitrosamine are far in excess of FDA’s established permissible daily intake limit for the probable human carcinogen.
David Light, CEO of Valisure, has stated:
“There’s no acceptable cancer risk for a drug like this.”
On October 2, 2019 the FDA issued a modest reversal:
“To date, the agency’s early, limited testing has found unacceptable levels of NDMA in samples of ranitidine. The agency will provide more information as it becomes available.”
On April Fool’s Day, 2020, the FDA finally announced:
“The U.S. Food and Drug Administration today announced it is requesting manufacturers withdraw all prescription and over-the-counter (OTC) ranitidine drugs from the market immediately. This is the latest step in an ongoing investigation of a contaminant known as N-Nitrosodimethylamine (NDMA) in ranitidine medications (commonly known by the brand name Zantac). The agency has determined that the impurity in some ranitidine products increases over time and when stored at higher than room temperatures and may result in consumer exposure to unacceptable levels of this impurity. As a result of this immediate market withdrawal request, ranitidine products will not be available for new or existing prescriptions or OTC use in the U.S.”
It is unlikely the FDA or any other organization will pay for long-term research to determine whether patients exposed to ranitidine were more likely to develop cancer.
Other Medicines and a Cancer Risk:
Humira (Adalimumab):
There are dozens of other drugs that come with a cancer warning. The highly promoted drug Humira (adalimumab) is prescribed for rheumatoid arthritis, colitis and psoriasis.
It comes with a black box warning:
“Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers including HUMIRA…These cases have had a very aggressive disease course and have been fatal.”
Other immune modulators such as Enbrel (etanercept) and Remicade (infliximab) also have malignancy warnings.
Actos (Pioglitazone) and Bladder Cancer Risk?
Pioglitazone was once a top-selling diabetes drug. It is estimated that the manufacturer, Takeda Pharmaceutical, took in over $20 billion since the drug’s approval in 1999.
Then it paid out $2.4 billion to patients or their families because of lawsuits. The issue: bladder cancer. Although the drug was banned in France, Germany and India, it is still on sale in the U.S.
The FDA does require this warning, though:
“Bladder cancer: May increase the risk of bladder cancer. Do not use in patients with active bladder cancer. Use caution when using in patients with a prior history of bladder cancer.”
What is a patient to make of such a cancer risk?
You can read more about the Actos controversy at this link:
Actos Maker Settles Lawsuits Worth Billions but Keeps Selling Drug Linked to Cancer
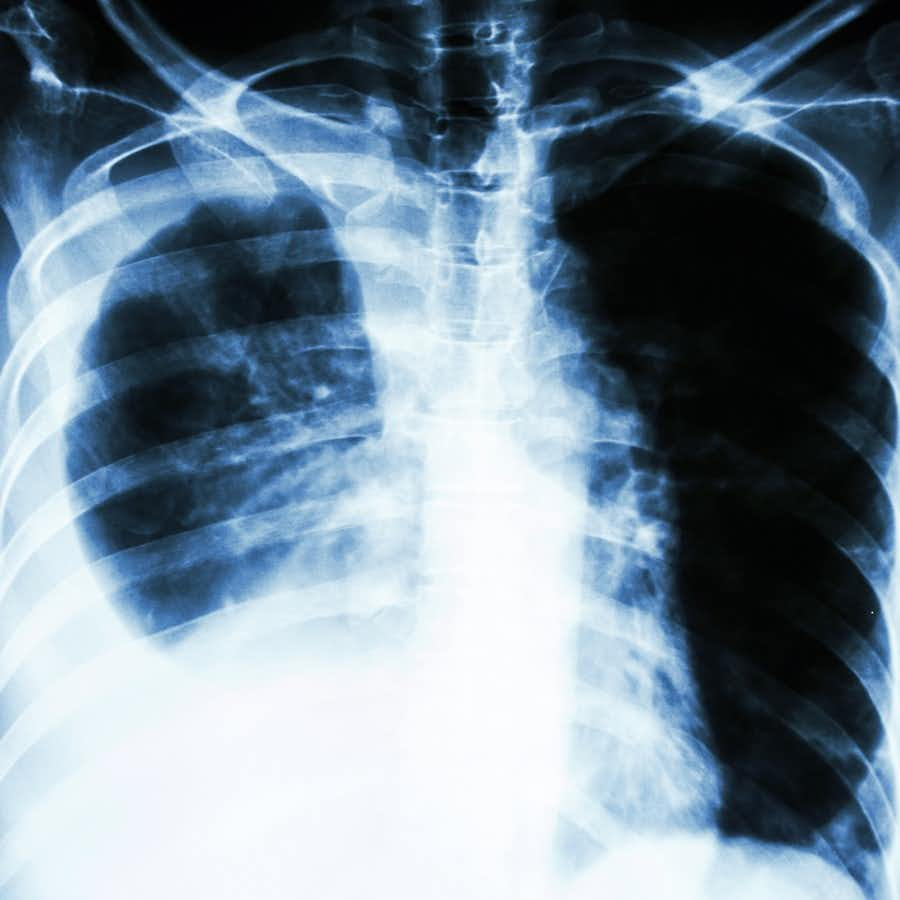
ACE Inhibitors and Lung Cancer?
It is estimated that as many as 30 million Americans take ACE inhibitors each year. ACE stands for angiotensin converting enzyme. These are drugs like benazepril (Lotensin), captopril (Capoten), enalapril (Vasotec), lisinopril (Prinivil, Zestril), (ramipril (Altace) and quinapril (Accupril).
Such drugs are very effective for controlling blood pressure. With the contamination controversy swirling around sartans, some doctors have switched patients to ACE inhibitors.
In 2012 there was a large (1.2 million) population-based study that reported a “modest increase in the risk of lung cancer” associated with ACE inhibitors (PLoS One, online, Dec. 12, 2012). Six years later another epidemiological study analyzed data from nearly one million patients (BMJ, Oct. 24, 2018).
The objective:
“To determine whether the use of angiotensin converting enzyme inhibitors (ACEis), compared with use of angiotensin receptor blockers [ARBs, aka “sartans”], is associated with an increased risk of lung cancer.”
The authors introduced their research this way:
“Angiotensin converting enzyme inhibitors (ACEIs) are effective drugs used in the treatment of hypertension. Although these drugs have been shown to be relatively safe in the short term, concerns have been raised that their long term use may be associated with an increased risk of cancer. These concerns have been subject to debate, with observational studies producing mixed findings, including with respect to lung cancer.”
Their Conclusions About a Cancer Risk:
“In this population based cohort study, the use of ACEIs was associated with an increased risk of lung cancer. The association was particularly elevated among people using ACEIs for more than five years. Additional studies, with long term follow-up, are needed to investigate the effects of these drugs on incidence of lung cancer.”
The cancer risk was considered “modest.” There was a “14% increased risk of lung cancer.” In terms of absolute risk: It went from 1.2 people per 1000 person-years for people not taking an ACEi to 1.6 people per 1000 person-years for those on an ACEi. That may seem less than “modest,” but when you consider how many millions of people are taking such drugs the total number potentially impacted is not trivial.
To make matters even more complicated, another meta-analysis was published in the journal Medicine (April 30, 2021).
It concluded:
“The use of ACEIs was not associated with an increased risk of lung cancer. Nevertheless, well-designed observational studies with different ethnic populations are still needed to evaluate the long-term (over 10 years) association between ACEIs use and lung cancer.”
Will anyone conduct such a long-term study? We find it doubtful that drug companies will spend the money to do this kind of research. The FDA rarely conducts this kind of investigation. We suspect that regulators and pharmaceutical manufacturers will wait for health professionals and patients to forget about any cancer risk controversy and try to pretend there is nothing to be concerned about.
No one should EVER stop taking any medication without medical consultation. ACE inhibitors are valuable drugs and the cancer risk, if it exists at all, needs to be confirmed. You can read more about this controversial topic at this link.
Hydrochlorothiazide (HCTZ) and Skin Cancer?
Here’s one that we doubt your doctor has ever heard of. The diuretic hydrochlorothiazide (abbreviated HCT or HCTZ) is taken by at least 20 million people. It is often combined with other blood pressure medications to exert a more powerful effect. We have written an article about the association between HCTZ and skin cancer:
Hydrochlorothiazide Side Effects: Skin Cancer and More!
What Should We Do About Drugs and a Cancer Risk?
We have only scratched the surface of this controversial topic. There are scores of medications people take every day that have some association with a cancer risk. Of course, that means that thousands of drugs do not have a cancer risk.
A cancer risk leaves both physicians and patients confused. Are patients in danger–or are these alerts meaningless? Without long-term research, we will probably never have a good answer. These controversies leave millions of people in limbo.
Drug Company Reassurance:
When pharmaceutical manufacturers announce a recall because of nitrosamine contamination, they frequently add a statement that goes something like this:
Drug Company XYZ “…has not received reports of adverse events related to the impurities or the recall” of our product.
Do you find such a statement reassuring? It usually shows up within weeks of a recall. Of course, since it can take many years for cancer to develop, such reassurances ring hollow to us.
If you watch drug commercials on TV you will hear warnings about lymphoma and other malignancies associated with medicines for rheumatoid arthritis, psoriasis and inflammatory bowel disease. Such warnings put patients and doctors in a double bind. It is time for the FDA to clarify the risk instead of just asking patients to ignore the possibility of cancer linked to their medicine.
Share Your Thoughts:
What do you think about concerns linking popular medications to a cancer risk? Is this a tempest in a teapot or something worrisome? Should the FDA require long-term studies to try to determine whether there is a meaningful risk?
Who should pay for such research? The original pharmaceutical companies are unlikely to pony up since many of their drug patents have expired. What should we do as a society to identify a cancer risk? The FDA seems reluctant to take much action.
If you think you are immune to such concerns because you do not take medications for hypertension, rheumatoid arthritis or diabetes, what about sunscreen, antiperspirants or deodorants? Some of these products have also been shown to have contaminants such as benzene, a known carcinogen. You can learn more about this controversy and ways to avoid contaminants in body care products at this link.
Please share your thoughts in the comment section below. If you think this article is worth sharing, please send it to family and friends by scrolling to the top of the page and clicking on the links for email, Facebook or Twitter. We would be grateful if you encouraged friends and family to subscribe to our free newsletter at this link.
Citations
- Azoulay, L., et al, "Long-term use of angiotensin receptor blockers and the risk of cancer," PLoS One, online, Dec. 12, 2012, doi: 10.1371/journal.pone.0050893
- Hicks, B.M., et al, "Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study," BMJ, Oct. 24, 2018, https://doi.org/10.1136/bmj.k4209
- Sipahi, I. "Risk of cancer with angiotensin-receptor blockers increases with increasing cumulative exposure: Meta-regression analysis of randomized trials," PLoS One, March 2, 2022, DOI: 10.1371/journal.pone.0263461