Doctors, pharmacists and nurses are directly responsible for patient care. They prescribe, dispense and oversee treatment. Why aren’t they more concerned about generic drug quality? We are confounded by the seeming indifference of health professionals to patients’ complaints about generic drug quality. Now, a new book, Bottle of Lies, confirms that there are huge gaps in FDA’s generic drug approval and monitoring process.
Why Aren’t Physicians and Pharmacists More Like Pilots?
When two separate Boeing 737 Max 8 airplanes crashed within several months of each other, the world took notice. Foreign regulators grounded this aircraft almost immediately. Pilots were outspoken in their criticism of the process that had allowed such a plane in the air.
Over the last year, manufacturers have recalled tens of millions of blood pressure pills. That’s because drugs like losartan, valsartan and irbesartan were found to be contaminated with carcinogens. The Chinese and Indian manufacturers had apparently been producing substandard products for years.
We have not heard an outcry from American health professionals. Doctors, nurses and pharmacists are responsible for their patients’ safety just as pilots are responsible for their passengers’ safety. Yet providers have not demanded changes in the way the Food and Drug Administration oversees the generic drug approval and monitoring process.
FDA’s Definition of Generic Drugs
For years, the FDA maintained that:
“A generic drug is identical, or bioequivalent to a brand name drug in dosage form, safety, strength, route of administration, quality, performance characteristics and intended use.” in Applied Health Economics and Health Policy, June 20, 2015. The same description is found on Medscape: “‘Generic Equivalent’: What Does That Really Mean?” (Oct. 2, 2017)
We pointed out to the FDA that the Oxford Dictionary defines the word identical as:
“adj. 1 agreeing in every detail. 2 one and the same.”
The Merriam-Webster Dictionary offers much the same definition:
“1: being the same 2: having such close resemblance as to be essentially the same.”
FDA Generic Drug Approval: Not Really Identical!
In truth, generic drugs are far from identical to their brand name counterparts. For one thing, brand name manufacturers do not hand over the recipe for making their medications. Generic drug companies have to reverse engineer products they want to copy. They often use different “excipients.” Those are the fillers, binders and coloring agents that hold the active pharmaceutical ingredient (API) together in the pills or capsules.
The formulation itself has to be recreated from scratch. When it comes to timed-release tablets, the generic manufacturer often has to develop a whole new technology.
The Food and Drug Administration has dropped the word “identical” from its current definition. Now the FDA defines a generic drug this way:
“A drug product that is comparable to a brand/reference listed drug product in dosage form, strength, route of administration, quality and performance characteristics, and intended use.”
In our opinion, “comparable” is not the same thing as “identical.” Since we put up such a fuss about the word identical, the FDA also uses this hard-to-understand definition (CFR – Code of Federal Regulations Title 21, April 1, 2018):
“Bioequivalence is the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.”
We think this definition muddies the waters significantly. We find the phrase “absence of a significant difference in the rate and extent…” gives the FDA a tremendous amount of leeway. We think this newer definition is way too ambiguous.
FDA’s Position: Be Happy, Don’t Worry!
Several years ago, we alerted the FDA to a large number of complaints about Budeprion XL 300, a branded generic form of the antidepressant Wellbutrin XL 300 (bupropion). Initially the FDA was skeptical. Officials insisted that any problems with Budeprion XL 300 were probably caused by psychosomatic factors. In other words, they were all in patients’ heads. Nothing to worry about.
We did not stop speaking up for patients, however. Eventually the agency decided to investigate. Independent testing showed that Budeprion XL 300 was not actually equivalent to brand name Wellbutrin XL 300. Other generic forms of bupropion also failed the tests.
Wellbutrin vs Budeprion Graphs
Read about this scandal at this link and see the so-called bioequivalence curves. You will find that the graphic comparing generic Budeprion 150 to Wellbutrin 150 shocking. The FDA still says these drugs are bioequivalent. This is not the same rate of absorption!
Do Physicians and Pharmacists Understand the Generic Drug Approval Process?
Most health professionals have no idea how the FDA’s generic drug approval system works. They just assume that the FDA knows what it is doing. But take a look at the graphs below and tell us that these drugs have the same rate of absorption.
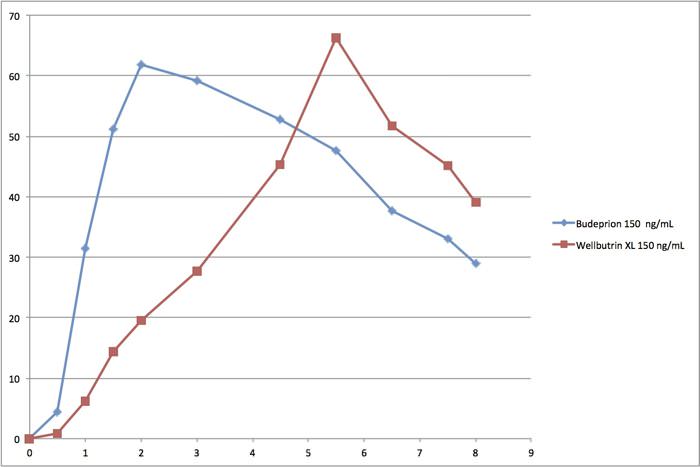
Note that the horizontal x-axis at the bottom is hours. Clearly, the generic Budeprion is absorbed into the body a lot faster than the brand name Wellbutrin. The generic peaks in less than 2 hours. The brand doesn’t peak until nearly 6 hours. Not identical!
What About OTHER Problems with Generic Drug Approval?
A new book by Katherine Eban reveals that there are huge problems with both the manufacture and regulation of many generic drugs. In her book, Bottle of Lies, Ms. Eban documents cases of fraud, shoddy manufacturing practices and regulatory failures. This book should be required reading for all health professionals.
One of the critiques of the Federal Aviation Administration (FAA) is that it relies too heavily on the manufacturers’ testing and certification. The same might be said for the FDA. The agency depends almost completely on test results supplied by drug makers.
If the data are manipulated, the FDA might have difficulty detecting that the medications are substandard. This may mean that some generic drugs are ineffective or unsafe.
Pilots Are Speaking Out! Where are the Physicians and Pharmacists?
Pilots have spoken up about their dismay with FAA’s handling of the Boeing 737 Max 8 crisis. One pilots’ union, the European Cockpit Association, declared that it was “extremely worrying” that “the manufacturer and the authorities are difficult to distinguish” in the FAA’s certification system.
The union president continued:
“What has been revealed is an oversight and regulatory setup that leaves pilots’ trust and confidence severely undermined.”
Safe and effective drugs, like safe and effective airplanes, require regulatory oversight independent of the manufacturers. Why haven’t physicians and pharmacists noticed problems with the quality of the drugs they prescribe and dispense every day? Why aren’t they as outraged as the pilots who are calling for changes in the way the FAA regulates airplanes?
The FDA often says it can’t change its procedures without legislative direction. Perhaps prescribers and dispensers (doctors and pharmacists) should be asking their senators and representatives to read Bottle of Lies. They should also read it themselves!
If you would like to listen to our free interview with Katherine Eban about the generic drug approval and monitoring process, here is a link to our radio show. Click on the green arrow above Katherine’s photo for streaming audio. You can also download the free mp3 file at the this link if you select that option or you could purchase a CD to give to your busy health professional.
Share Your Thoughts:
Why do you think health professionals have remained silent about this scandal? Have you had personal experience with the generic drug approval or monitoring process? We would love to get your perspective in the comment section.