The brand new report from ProPublica reads like a detective story. A whistleblower working for a drug testing firm alerts the FDA that some scientists in the company were fudging data. A team of FDA investigators arrives at Cetero Research in Houston on May 3, 2010 and confronts the president with the allegations. He admits, “You got us.”
If this scenario were in a spy novel, the revelation would be followed by full disclosure of who the bad guys were, what they did wrong, which drugs were involved and how the FDA planned to right the wrongs. Unfortunately, in real life this scandal was not publicized. Without ProPublica’s recent expose, we would never have heard about Cetero’s data falsification.
It turns out that between April 2005 and August 2009 the fraud at the firm was so “egregious” that the FDA could not trust the results of any of the studies done then. Roughly 100 drugs are affected, over 80% of them generics.
You might assume that the FDA would have immediately notified the American public which drugs were under a cloud. In fact, you might think that the FDA would have suspended sales of these products until other testing could confirm that they met the agency’s standards. But you would be wrong.
To our amazement, the FDA to this day has never released the names of the affected drugs. Their rationale is that this is “confidential commercial information.” What? Shouldn’t the health of the American public trump commercial interests, especially if questions about quality remain unanswered? As a consequence we have no idea which generic medications are really “identical” to their brand name counterparts and which ones might fall short.
This question has troubled us for some time. Over the last decade we have become increasingly concerned about the FDA’s approval process for generic drugs and the agency’s ability to monitor quality of such products. The reason for our uneasiness grew out of the thousands of complaints we received on our website (www.PeoplesPharmacy.com) about generic drug problems. Initially we did not know quite what to make of these grumbles. After all, we had been enthusiastic supporters of generic drugs for more than two decades. But eventually the chorus of complaints grew to such an extent that we could no longer ignore the stories.
The straw that broke the camel’s back occurred over six years ago when we started receiving reports of serious complications linked to the generic antidepressant formulation for Wellbutrin XL 300 called Budeprion XL 300 (bupropion). Readers of our syndicated newspaper column began sharing painful stories about distressing side effects that appeared when they were switched to the generic. J. in Dansville, New York, wrote to say: “I have been taking Budeprion XL 300 mg for three months instead of Wellbutrin XL 300 mg. I find that I am easily upset and cry very easily. Sometimes I feel aggressive. I also have short, stabbing pains in my head.” Others reported nausea, insomnia, tremors, anxiety and suicidal thoughts.
Eventually, so many problems were reported to the FDA that it asked the generic drug maker, Teva, to do another study. That was never completed, but the agency ultimately commissioned its own research that determined that Budeprion XL 300 was not bioequivalent to Wellbutrin XL 300. The Budeprion product was pulled from the market in October, 2012.
That wasn’t soon enough for one unfortunate patient. Andrew Richards was switched from Wellbutrin XL 300 to Budeprion XL 300 and soon after that, in March 2008, he had a seizure. Read the ProPublica story. It felt, he said, like a lightning bolt that brought him from his sofa to the floor. Although he is no longer taking the medication, he continues to be troubled by the aftermath of that event. He won a lawsuit against Teva.
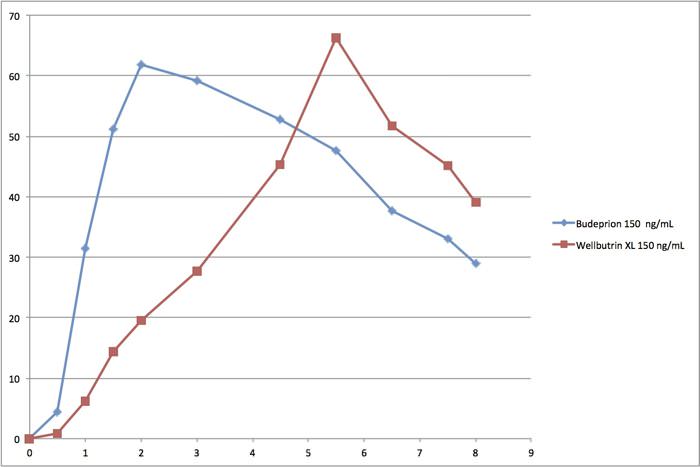
A|though Budeprion does not seem to be one of the drugs tested by Cetero Research, it is so far the only generic medicine that has been taken off the market for not meeting bioequivalence requirements. How many other generic medications might be in the same situation? We have begged the FDA to look at the lower-dose Budeprion 150. Plotting the agency’s own data yields this graph comparing the generic to the brand-name compound:
In this graph, the X axis (horizontal) is measured in hours, while the Y axis (vertical) is measured in ng/ml, a measure of how much medication is in the blood stream. Although the FDA considers these two lines to be “identical,” they don’t look identical to us. We think that FDA should take a second look at this medicine, but the agency may be so busy reviewing problems uncovered in the Cetero scandal that it doesn’t have time to scrutinize this. You may be surprised to learn that Budeprion XL 150 (the generic drug in the graph above) remains on the market to this day and the FDA maintains that it is fine and dandy, ie, bioequivalent to the brand name Wellbutrin XL 150. People would take this twice or three times a day according to the official prescribing information.
The Bottom Line:
The Cetero scandal is unlikely to have been uncovered had it not been for an honest employee who blew the whistle. We don’t know about you, but we now worry if there aren’t other testing laboratories out there that may not be meeting rigorous scientific standards. And what about foreign manufacturing and testing? If the FDA can’t discover such problems here in the U.S., we have little hope that the agency can uncover fraud in countries where scientific integrity may not be as highly regarded.
The FDA inspector in Houston who unraveled the Cetero mess was Patrick Stone (since retired). Here is the conclusion from the ProPublica article:
“The FDA’s Stone draws little satisfaction from unraveling the problems at Cetero.
“There are thousands of bioequivalence studies done every year, he pointed out, with each study generating thousands of pages of paper records. ‘Do you really think we’re going to look at 100 percent of them? We’re going to look at maybe 5 percent if we’re lucky,’ he said. ‘Sometimes 1 percent.’
“Still, given how often he and other FDA teams had inspected the Houston lab, he thinks regulators should have spotted Cetero’s misconduct sooner.
“‘In hindsight I look back and I’m like, ‘Wow, should I be proud of this?'” he said. ‘It’s cool that I was part of it, but it’s crap that we didn’t catch it five years ago. How could we let this go so long?'”
If you are interested in problems with generic drugs, here are links to experiences other readers have shared.
If you are as outraged as we are, please contact the FDA and your Congressional representative. At the very least, demand that the FDA release the names of the drugs implicated in the Cetero scandal. Share your own story below.